EU Clinical Trial Regulation 536/2014: Procedure and Timelines
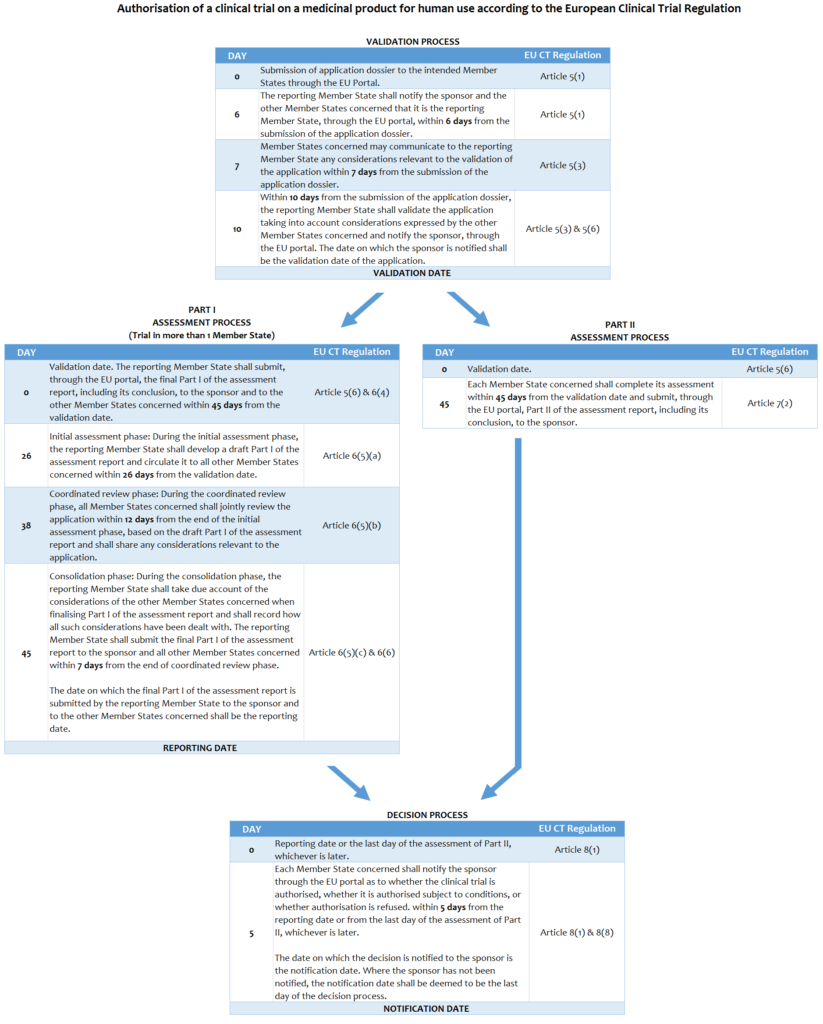
Bontrop, Vincent (2015): EU Clinical Trial Regulation: Procedure and Timelines. figshare. http://dx.doi.org/10.6084/m9.figshare.1326450
The texts are derived from the European Clinical Trial Regulation No.536/2014, and (if necessary) modified for instructional and educational purposes.
References
- Bontrop, V. (2015). EU Clinical Trial Regulation: Procedure and Timelines. figshare. http://dx.doi.org/10.6084/m9.figshare.1326450
- Regulation No 536/2014 of the European Parliament and of the Council on clinical trials on medicinal products for human use, and repealing Directive 2001/20/EC http://ec.europa.eu/health/files/eudralex/vol-1/reg_2014_536/reg_2014_536_en.pdf
