Uitgelicht en gespot op internet (week 37, 2023)
Hierbij een nieuwe uitgave van ‘Uitgelicht en gespot op internet‘, een supplement van de ‘Nieuwsbrief voor Goede Onderzoekspraktijken‘, met een overzicht van nieuws en andere berichten die zijn gespot op het internet.
Vincent Bontrop
www.bontrop.com
U kunt met mij linken via LinkedIn.
Algemene verordening gegevensbescherming (AVG), Health-RI
Clinical Trials Coordination Group (CTCG)
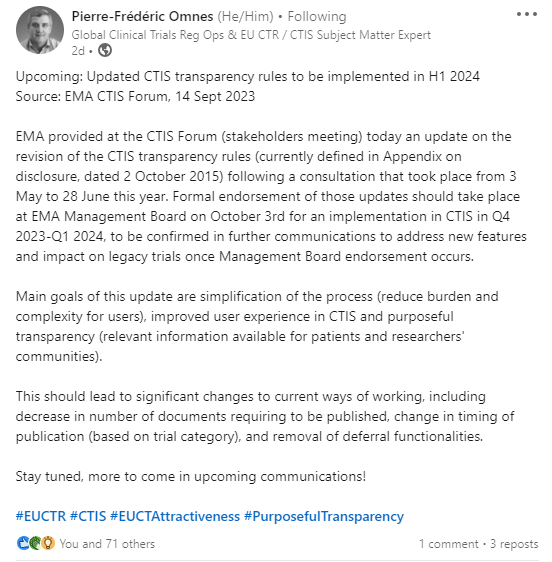
Clinical Trials Regulation (CTR), Clinical Trials Information System (CTIS)
Nederlandse Vereniging voor Farmaceutische Geneeskunde (NVFG)
|
DARWIN-EU
European Medicines Agency (EMA), GCP, GLP, GMP
- Compliance: Overview – “All organisations involved in the development, marketing, manufacture and distribution of medicines are responsible for ensuring that they comply with all relevant standards set out in European Union (EU) legislation and guidelines on pharmaceuticals. The European Medicines Agency (EMA) is responsible for harmonising these standards at EU level. It also coordinates inspections to verify compliance.“
- Towards a permanent collaboration framework for EMA and Health Technology Assessment bodies (15-09-2023).
- Minutes of the 120th meeting of the Management Board: 7-8 June 2023 (15-09-2023).
Inspectie Gezondheidszorg en Jeugd (IGJ)
 Kunstmatige Intelligentie (KI)
Kunstmatige Intelligentie (KI)
- A Review of Medical A.I Randomized Trials (15-09-2023). Ground Truths.
- Inside the “black box”: Embedding clinical knowledge in data-driven machine learning for heart disease diagnosis. Cardiovasc Digit Health J. 2022 Nov 2;3(6):276-288. doi: 10.1016/j.cvdhj.2022.10.005. PMID: 36589311; PMCID: PMC9795264.
| Medical Device Regulation (MDR), In Vitro Diagnostic Medical Device Regulation (IVDR)
Centrale Commissie Mensgebonden Onderzoek (CCMO) Post-Market Surveillance (PMS) |
Medicines and Healthcare products Regulatory Agency (MHRA)
- Guidance MHRA phase I accreditation scheme (updated list of Accredited Units_15 September 2023).
- Clinical Trials Performance Metrics: September 2022 – August 2023: Assessment of Clinical Trial Authorisation Applications and Substantial Amendments.
Nieuwsbrief voor Goede Onderzoekspraktijken
Nederlandse Federatie van Universitair Medische Centra (NFU)
- Richtlijn Kwaliteitsborging Mensgebonden Onderzoek 2023
- Guideline Quality assurance of research involving human subjects 2023
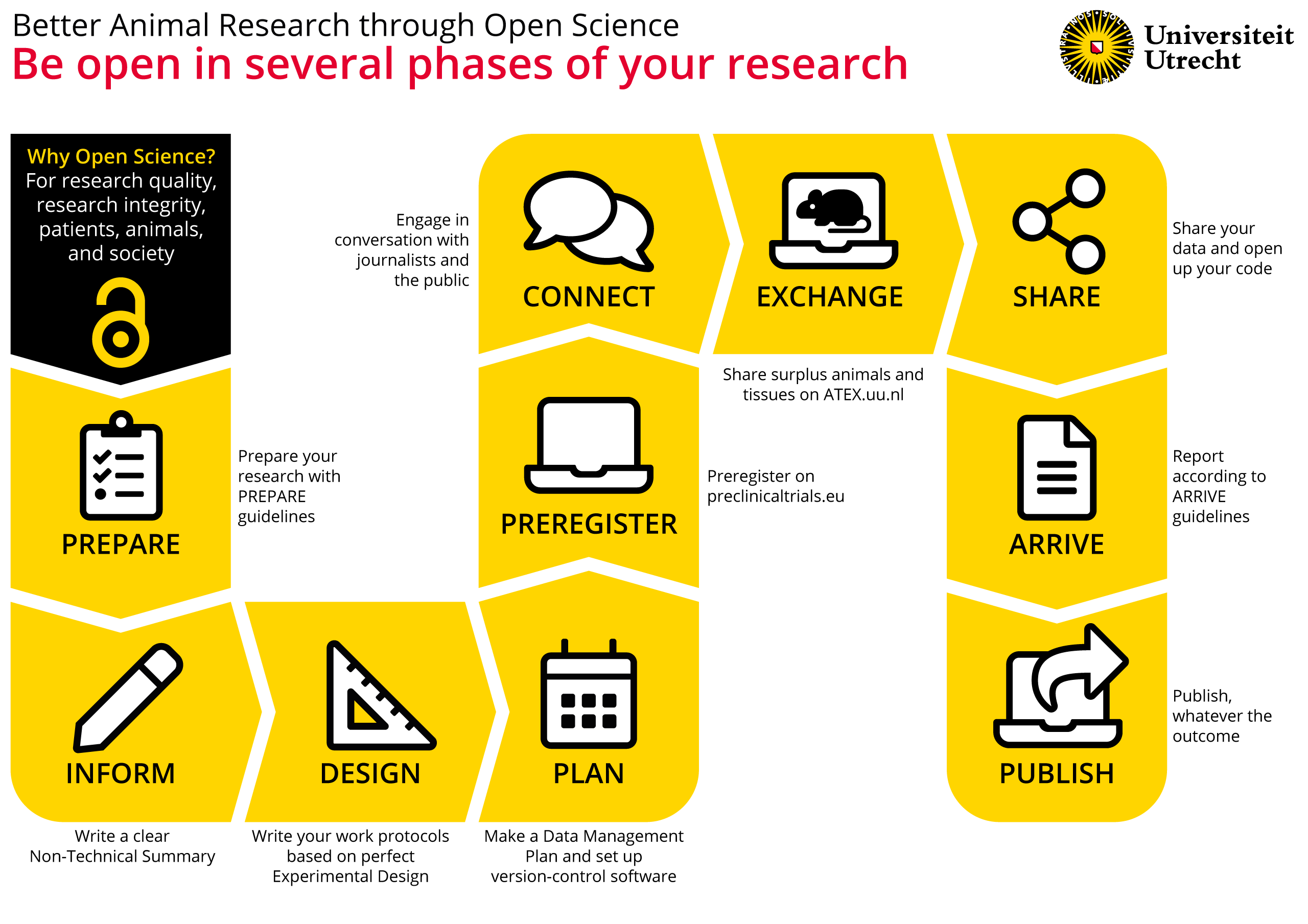
Open Science
Transparantieregister Zorg, Governance financiële relaties
- Vijf tips voor een goede registratie in het Transparantieregister Zorg. Maak je registratie steekproef-proof! (13-09-2023). Marloes Meddens-Bakker, via LinkedIn.
- Handreiking Governance financiële relaties zorgprofessionals en industrie (herziene versie 2.0) (06-09-2023). NVZ, FMS & NFU.
Uit het Archief, Historie
- CCMO-rapport Toetsing CRISP-studie door METC Erasmus MC + brief CCMO aan voorzitters METC’s (08-10-2019).
- CCMO-rapport Toetsing DRAINAGE-studie METC Academisch Medisch Centrum Amsterdam (26-07-2018).
- Overkoepelend rapport PROPATRIA-studie (01-12-2009). IGZ, CCMO, VWA.
- CCMO‐rapport PROPATRIA‐studie (01-11-2009).
- Handreiking IGZ: voedings- of geneesmiddel? (17-12-2009). IGZ.
US Food and Drug Administration’s (FDA), National Institutes of Health (NIH)
- Draft guidance: Medical Devices with Indications Associated with Weight Loss – Clinical Study and Benefit-Risk Considerations: Draft Guidance for Industry and Food and Drug Administration Staff(15-09-2023).
- Medical Devices with Indications Associated with Weight Loss – Non-Clinical Recommendations: Draft Guidance for Industry and Food and Drug Administration Staff (15-09-2023).
- Draft guidance: Clinical Pharmacology Considerations for Peptide Drug Products (11-09-2023).
- Final guidance: Institutional Review Board (IRB) Review of Individual Patient Expanded Access Submissions for Investigational Drugs and Biological Products: Guidance for IRBs and Clinical Investigators (11-09-2023).
- Draft guidance: Endogenous Cushing’s Syndrome: Developing Drugs for Treatment (08-09-2023).
- Navigating Compassionate Use in Clinical Research: Unpacking FDA’s Latest Guidance (14-09-2023). Clinical Trial Vanguard.
- FDA’s New RWE Guidance Provides Recommendations for Sponsors Conducting Non-Interventional Real-World Studies and Describes the Potential to Use RWE for Initial Approvals in One Limited Circumstance (11-09-2023). FDA Law Blog.
- Recommendations on responsible stewardship of personal health data in clinical research (29-09-2023).
Overig
- Weekend reads: Crossref acquires the Retraction Watch Database; Italy minister’s papers scrutinized; Carlo Croce goes to court yet again (16-09-2023). Retraction Watch.
- Randomized controlled trials are the ‘gold standard’ of research — but a difficult fit for trans care (15-09-2023). STAT.
- Human trials of artificial wombs could start soon. Here’s what you need to know (14-09-2023). Nature.
- Bedrijven sponsoren universiteiten en dat is goed (14-09-2023). NRC.
- A case of so-called fiscal neutrality (12-09-2023). Medicaldeviceslegal.
- The Key Information Section in Consent – Why, What, and How (12-09-2023). Learn eCORE.
- De Route naar databeschikbaarheid (11-09-2023). ICT&health.
- Motivations for paediatric vaccine trial participation. Trials 24, 574 (2023).
Op de hoogte blijven van actueel nieuws, bijeenkomsten en publieke consultaties