Uitgelicht en gespot op internet (week 36, 2023)
Hierbij een nieuwe uitgave van ‘Uitgelicht en gespot op internet‘, een supplement van de ‘Nieuwsbrief voor Goede Onderzoekspraktijken‘, met een overzicht van nieuws en andere berichten die zijn gespot op het internet.
Vincent Bontrop
www.bontrop.com
U kunt met mij linken via LinkedIn.
Algemene verordening gegevensbescherming (AVG), European Data Protection Board (EDPB)
| Clinical Trials Regulation (CTR), Clinical Trials Information System (CTIS)
Integraal Kankercentrum Nederland (IKNL) |
Dierproeven
- Colombia considers ban on most research and education using live animals (07-09-2023). Nature.
- Aantal dierproeven Universiteit Utrecht en UMC Utrecht opnieuw vrijwel gelijk gebleven (04-09-2023). UMC Utrecht.
Diversity, Inclusivity
- What could fix cancer clinical trials’ longstanding diversity problem? (07-09-2023). STAT.
- Conducting inclusive research in genetics for transgender, gender-diverse, and sex-diverse individuals: Case analyses and recommendations from a clinical genomics study (04-09-2023). Journal of Genetic Counseling.
European Medicines Agency (EMA)
- Draft guideline on clinical investigation of medicinal products in the treatment of depression – Revision 3 (07-09-2023).
- DRAFT qualification opinion for GFR slope as a Surrogate Endpoint in RCT for CKD (06-09-2023).
Fraud, Misconduct, Integrity
- Weill Cornell cancer researchers committed research misconduct, feds say (07-09-2023). Retraction Watch.
- Evidence of questionable research practices in clinical prediction models. BMC Med 21, 339 (2023).
- Flawed chemistry papers can take more than a year to be retracted (28-08-2023). C&EN.
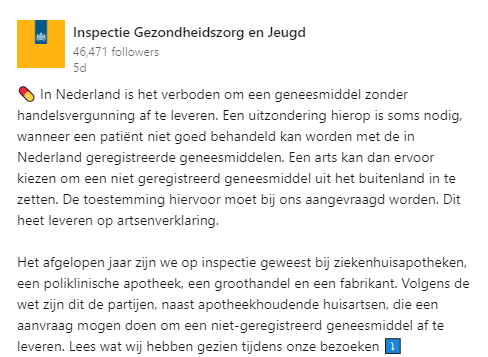
Inspectie Gezondheidszorg en Jeugd (IGJ), Leveren op artsenverklaring
- Factsheet Geneesmiddelen op artsenverklaring: een noodzakelijke uitzondering (04-09-2023). IGJ.
- Leveren op artsenverklaring. IGJ.
- IGJ publiceert factsheet over geneesmiddelen op artsenverklaring (07-09-2023). KNMP.
- IGJ: levering op artsenverklaring niet altijd volgens de regels (05-09-2023). PW.
International Federation of Associations of Pharmaceutical Physicians and Pharmaceutical Medicine (IFAPP)
Kunstmatige Intelligentie (KI)
- AI is here to stay. What does it mean for medicine regulation? (06-09-2023). Emer Cooke, via LinkedIn.
- Ethical and legal framework for AI applications in drug development of ‘utmost importance’ (06-09-2023). Lygature.
- De AI Act vraagt een nieuwe pet: de AI Compliance Officer (06-09-2023). ICTRecht.
- The ethics of ChatGPT – Exploring the ethical issues of an emerging technology (2024). International Journal of Information Management.
Medicines and Healthcare products Regulatory Agency (MHRA)
Methodologie, Statistiek, Gegevenswetenschappen (Datascience)
- Evaluation of randomized controlled trials: a primer and tutorial for mental health researchers. Trials 24, 562 (2023).
- Blog 22A: Statistics and Data Science – The Two Cultures (06-09-2023). Analytix Thinking.
- Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement (26-10-2021). JAMA. 2021;326(16):1614–1621. doi:10.1001/jama.2021.18236
Rare diseases, Weesziekten (Orphan diseases)
- Toward responsible clinical n-of-1 strategies for rare diseases. Drug Discov Today. 2023 Jun 24:103688. doi: 10.1016/j.drudis.2023.103688. Epub ahead of print. PMID: 37356616.
- The Regulatory Repercussions of Approving Muscular Dystrophy Medications on the Basis of Limited Evidence. Ann Intern Med. 2023 Aug 22. doi: 10.7326/M23-1073. Epub ahead of print. PMID: 37603868.
- The IRDiRC Chrysalis Task Force: making rare disease research attractive to companies (29-07-2023). Therapeutic Advances in Rare Disease.
- Methods to Increase Signal Finding in Rare Disease Drug Development (08-09-2023). Certara.
Regulatory Science Network Netherlands (RSNN)
Uit het Archief, Historie
US Food and Drug Administration’s (FDA)
- Draft guidance: Clinical Pharmacology Considerations for Peptide Drug Products (11-09-2023).
- Final guidance: Institutional Review Board (IRB) Review of Individual Patient Expanded Access Submissions for Investigational Drugs and Biological Products: Guidance for IRBs and Clinical Investigators (11-09-2023).
- Final guidance: Application of Human Factors Engineering Principles for Combination Products: Questions and Answers: Guidance for Industry and FDA Staff (07-09-2023).
- FDA finalizes guidance on use of human factors studies in combination product development (08-09-2023). Regulatory Focus.
Overig
- Weekend reads: ChatGPT in papers; a Russia-based paper mill; getting scooped becomes an opportunity (09-09-2023). Retraction Watch.
- In het lab werkte de medicatie. Maar de echte wereld is geen lab (10-09-2023). Trouw.
- Start of Oncode Accelerator: patients at the center of innovative cancer drug development (08-09-2023). Lygature.
- How a supplement company became a haven for health misinformation (08-09-2023). STAT.
- How to Review an Academic Journal Article (06-09-2023). Inside Higher Education.
- Response to Open Peer Commentaries on “Do Clinicians Have a Duty to Participate in Pragmatic Clinical Trials?” (05-09-2023). The American Journal of Bioethics.
- Duration of Effectiveness Evaluation of Additional Risk Minimisation Measures for Centrally Authorised Medicinal Products in the EU Between 2012 and 2021 (02-09-2023). Drug Safety.
- Optimizing data integration in trials that use EHR data: lessons learned from a multi-center randomized clinical trial. Trials 24, 566 (2023).
- Time for a proper career pathway for clinical trial managers?. Trials 24, 565 (2023).
- Food or Medicine? A European Regulatory Perspective on Nutritional Therapy Products to treat Inborn Errors of Metabolism. J Inherit Metab Dis. 2023 Aug 31. doi: 10.1002/jimd.12677. Epub ahead of print. PMID: 37650776.
- Report published on how socioeconomic data can contribute to infectious disease research (29-08-2023). DANS.
- The role of gender and coauthors in academic publication behavior (december 2023). Research Policy.
Op de hoogte blijven van actueel nieuws, bijeenkomsten en publieke consultaties