Uitgelicht en gespot op internet (week 51, 2022)
Hierbij een nieuwe uitgave van ‘Uitgelicht en gespot op internet‘, een supplement van de ‘Nieuwsbrief voor Goede Onderzoekspraktijken‘, met een overzicht van nieuws en andere berichten die zijn gespot op het internet.
Ook zijn er weer nieuwe boeken toegevoegd aan de Boekenplank. En vergeet niet om de Events- en de Consultations-pagina te bezoeken.
Veel leesplezier!
Vincent Bontrop
www.bontrop.com
U kunt met mij linken via LinkedIn en Mastodon.
Centrale Commissie Dierproeven (CCD)
- Jaarplan CCD 2023 (23-12-2022).
- Verslag vergadering 25 november 2022 (23-12-2022).
- Herziene handreiking genetisch gewijzigde dieren gepubliceerd (20-12-2022).
- Herziene handreiking genetisch gewijzigde dieren 1 januari 2023 (20-12-2022).
Clinical Trials, Clinical Research, Decentralized Clinical Trials, Drug Development
- Decentralized Guidance Is the Best That Europe Can Do for Decentralized Clinical Trials (22-12-2022). Applied Clinical Trials.
- Major German research funder launches audit of clinical trial portfolio (20-12-2022). TranspariMED.
- Advancing Use and Acceptance of Decentralized Clinical Trials with Digital Elements. DIA Global Forum.
- Successful DCTs: Design Thinking for Optimized Clinical Trials. DIA Global Forum.
- Approaches to prioritising research for clinical trial networks: a scoping review. Trials 23, 1000 (2022).
- Considerations when introducing electronic patient-reported outcome data capture in multicentre oncology randomised controlled trials. Trials 23, 1004 (2022).
- Assessing the acceptability of individual studies that use deception: A systematic review of normative guidance documents (14-12-2022). Accountability in Research.
- Clinical, methodology, and patient/carer expert advice in pediatric drug development by conect4children (12-12-2022). Clinical and Translational Science.
Clinical Trials Regulation (CTR)
- Key performance indicators (KPIs) to monitor the European clinical trials environment (1-30 November 2022, edition 8) (21-12-2022). EMA.
- Newsletter: Clinical Trials Highlights – December 2022 (21-12-2022). EMA.
- Use of new European registry for drug trials mandatory from February 2023 (21-12-2022). TranspariMED.
- CTIS – Status update: Implementation of the Clinical Trials Regulation.
- Clinical trials in human medicines. EMA.
- Facilitating Decentralised Clinical Trials in the EU (19-12-2022). EMA.
- Aandachtspunten speciaal voor geneesmiddelenonderzoek. IGJ.
Commissie Genetische Modificatie (COGEM)
- Risico’s voor mens en milieu bij gentherapieonderzoek: wat is aanvaardbaar? (21-12-2022).
- Commissie Genetische Modificatie (COGEM), 2020. Signalering Risico’s voor mens en milieu bij gentherapieonderzoek: Wat is aanvaardbaar? Cogem-signalering CGM/221206-02.
- COGEM Nieuwsbrief december 2022 (22-12-2022).
Diversity, Inclusion, Informed consent
- Informed Consent: Research Staff’s Perspectives and Practical Recommendations to Improve Research Staff-Participant Communication (23-12-2022). Journal of Empirical Research on Human Research Ethics.
- Inclusion of Pregnant and Lactating Persons in Clinical Trials: Proceedings of a Workshop. Washington, DC: The National Academies Press.
- Underrepresentation of women in randomized controlled trials: a systematic review and meta-analysis. Trials 23, 1038 (2022).
Dutch Association of Research Quality Assurance (DARQA)
- DARQA GLP themadag ““Diergeneesmiddelenonderzoek, GLP meets VICH GCP”, 2 december 2022 (19-12-2022).
European Federation of Pharmaceutical Industries and Associations (EFPIA)
European CRO Federation (EUCROF)
Federaal Agentschap voor Geneesmiddelen en Gezondheidsproducten (FAGG) (België)
Good Publication Practice (GPP)
Health-RI
- De gezondheid van morgen, begint vandaag (22-12-2022).
- Integrale wetgeving nodig voor medisch wetenschappelijk onderzoek (19-12-2022).
Healixia
In Vitro Diagnostic Regulation (IVDR)
International Council for Harmonisation (ICH)
- ICH M13 draft Guideline reaches Step 2 of the ICH process (21-12-2022).
“The ICH M13A draft Guideline on Bioequivalence for Immediate-Release Solid Oral Dosage Forms reached Step 2 of the ICH process on 20 December 2022.“
Investigational Medicinal Products (IMP), Good Clinical Practice (GCP), Good Manufacturing Practices (GMP)
KCE Trials
Kunstmatige Intelligentie (KI)
Landelijk Trial Register (LTR)
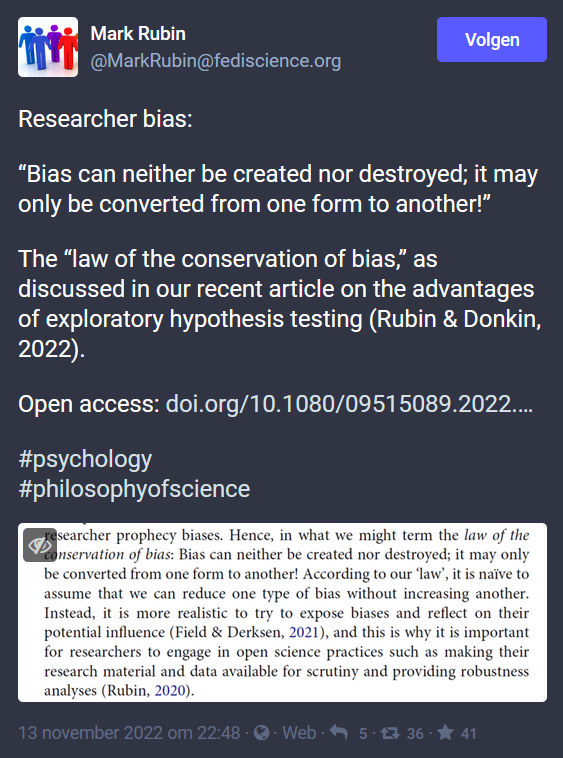
Methodology, Design, Statistics, Bias
- Duration of and time to response in oncology clinical trials from the perspective of the estimand framework (21-12-2022). arXiv:2212.10911.
- How do we know a treatment is good enough? A survey of non-inferiority trials. Trials 23, 1021 (2022).
- Composite Endpoint for Acceptability Evaluation of Oral Drug Formulations in the Pediatric Population. Ther Innov Regul Sci 56, 903–909 (2022).
- Surrogate endpoints in trials: a call for better reporting. Trials 23, 991 (2022).
- Sample size requirement in trials that use the composite endpoint major adverse cardiovascular events (MACE): new insights. Trials 23, 1037 (2022).
- Establishing a Core Domain Set for early-phase clinical trials of electrical stimulation interventions for tinnitus in adults: protocol for an online Delphi study. Trials 23, 1039 (2022).
Proefschriften
Quality of scientific research
Research Ethics, Research Integrity
- UCSF Issues Report, Apologizes for Unethical 1960-70’s Prison Research (20-12-2022). UCSF.
- Research Integrity Supervision Practices and Institutional Support: A Qualitative Study. J Acad Ethics (2022).
- Effect of medical researchers’ creative performance on scientific misconduct: a moral psychology perspective. BMC Med Ethics 23, 137 (2022).
- Journal editors and publishers’ legal obligations with respect to medical research misconduct (26-12-2022). Research Ethics.
- The ethics review and the humanities and social sciences: disciplinary distinctions in ethics review processes (26-12-2022). Research Ethics.
US Food and Drug Administration (FDA)
Xenotransplantatie
Overig
- Weekend reads: UCSF apologizes for prison research; top judge in Mexico accused of plagiarism; peer review under scrutiny (24-12-2022). Reatraction Watch.
- Weekend reads: Fringe race science and journals; flags for Stanford president’s papers; rise and fall of peer review (17-12-2022). Retraction Watch.
- Het veranderende biosimilar-landschap in de Verenigde Staten (23-12-2022). Biosimilars Nederland.
- Evaluation of New Active Substance Status for Biological Products in the EU (21-12-2022). BioSlice Blog, Arnold & Porter.
- Reporting guidelines for publishing health research (22-11-2022). EV Science Consultant.
- Medical racism didn’t begin or end with the syphilis study at Tuskegee (20-12-2022). Science News.
- Jumping over the paywall: Strategies and motivations for scholarly piracy and other alternatives (09-12-2022). arXiv:2212.05965.
- PDF´s vinden van wetenschappelijke publicaties (01-04-2019). EV Science Consultant.
- Here’s why we’re not prepared for the next wave of biotech innovation (03-11-2022). STAT.
- SURF en Universiteiten van Nederland werken aan pilot Mastodon voor het hele onderwijs (23-12-2022). Universiteiten van Nederland.
- Twitter beleid van Musk staat symbool voor een groter probleem (22-12-2022). Bits of Freedom.
- Dat Elon Musk Twitter heeft gekocht, moet ons aan het denken zetten (24-11-2022). Rathenau Instituut.
Op de hoogte blijven van actueel nieuws, bijeenkomsten en publieke consultaties