Uitgelicht en gespot op internet (week 47, 2022)
Hierbij een nieuwe uitgave van ‘Uitgelicht en gespot op internet‘, een supplement van de ‘Nieuwsbrief voor Goede Onderzoekspraktijken‘, met een overzicht van nieuws en andere berichten die zijn gespot op het internet.
Vergeet niet om ook regelmatig een kijkje te nemen op de Consultations-pagina (consultations.bontrop.com) en de Events-pagina (events.bontrop.com).
Vincent Bontrop
www.bontrop.com
U kunt met mij linken via LinkedIn en Mastodon.
Accelerating Clinical Trials in the EU (ACT EU)
- Accelerating Clinical Trials in the EU (ACT EU): Focus on Decentralized Clinical Trial Innovation (17-11-2022). Medidata.
- ACT EU state of play: EMA’s headway to transform clinical trials (15-11-2022). Clinical Trials Arena.
Algemene verordening gegevensbescherming (AVG), Data Privacy Impact Assessment (DPIA)
Centrale Commissie Dierproeven (CCD)
Clinical trials
- Leading clinical trials companies in the artificial intelligence theme (26-11-2022). Clinical Trials Arena.
- How Clinical Trials Can Thrive in the Digital Era (22-11-2022). Applied Clinical Trials.
- Voices of Women With Lived Experience of Substance Use During Pregnancy: A Qualitative Study of Motivators and Barriers to Recruitment and Retention in Research. Family & Community Health: January/March 2023 – Volume 46 – Issue 1 – p 1-12 doi: 10.1097/FCH.0000000000000349.
- De-risking DCTs with Quality Systems (22-11-2022). Applied Clinical Trials.
Clinical Trials Regulation (CTR)
Clinical trials results reporting
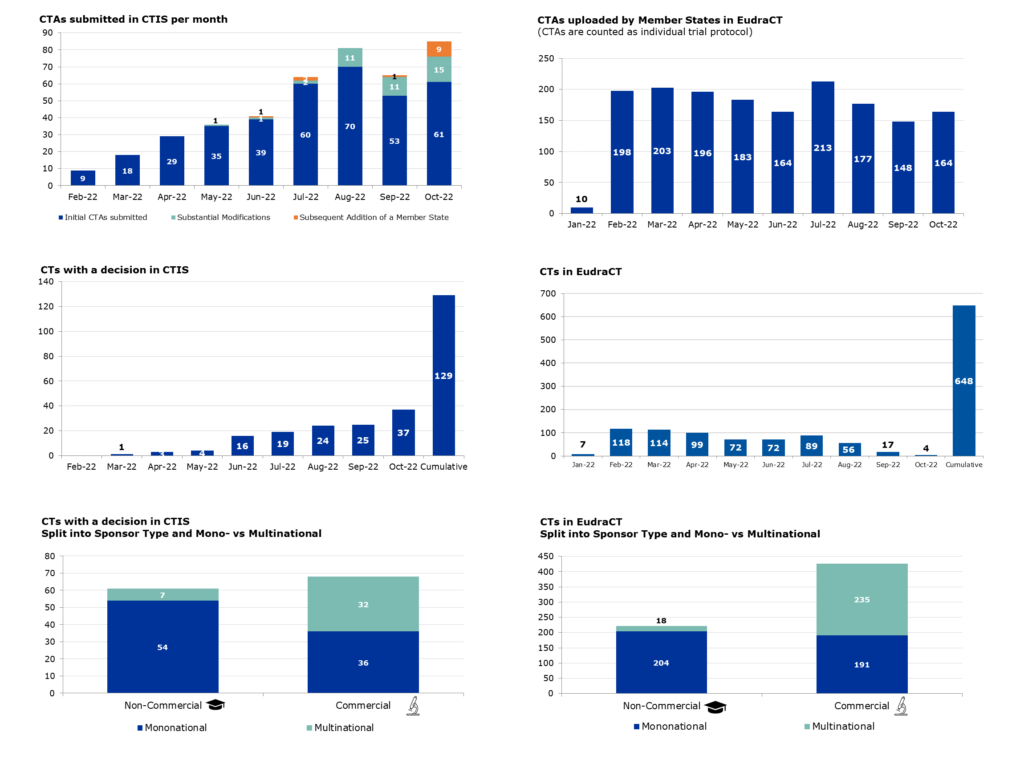
As of June 2022, 81% of clinical trials are compliant with this requirement in EudraCT.
Between April 2019 and June 2022, the compliance rate rose:
- from 24% to 64% for non-commercial sponsors;
- from 77% to 86% for commercial sponsors.
COVID-19
Data Analysis and Real-World Interrogation Network (DARWIN EU)
- DARWIN EU® welcomes first data partners (23-11-2022).
- DARWIN EU data partners onboarded in phase I (23-11-2022).
- IKNL datapartner in Darwin EU®-netwerk van het European Medicines Agency (24-11-2022). IKNL.
DARWIN EU collaborates with data partners who help generate real-world evidence that can be used in scientific evaluations and regulatory decision-making. The data partners enable DARWIN EU to use their data in its scientific studies, and provide analysis results to the DARWIN EU coordination centre, in accordance with data protection rules.
bron: EMA website ‘Data Analysis and Real World Interrogation Network (DARWIN EU)’
Data reuse
Decentralized Trials & Research Alliance (DTRA)
Dierexperimenteel onderzoek
Dutch Association of Research Quality Assurance (DARQA)
Dutch Oncology Research Platform (DORP)
EudraCT (European Union Drug Regulating Authorities Clinical Trials Database)
What’s New: From 31 January 2023, sponsors will need to use the Clinical Trials Information System to apply for authorisation of a new clinical trial in the EU/EEA. From this date onwards, EudraCT will no longer allow the creation of new EU/EEA Clinical Trial Application (CTA) for the purpose of submission to National Competent Authorities (NCAs), including creation of CTA(s) in order to add member state(s) to an existing EudraCT trial. See section 11 of the Q&A on Regulation (EU) 536/2014.
EudraCT will remain available to sponsors for: amendments of CTAs that were submitted to NCAs before January 31st, 2023; creation and uploading of PIP/Art 46 trials conducted exclusively in third countries (see FAQs); results submission for all EudraCT trials.
22-11-2022: Sponsors of clinical trials on COVID-19 are reminded to include the term “COVID-19” in the title of their trial (section A.3 of the CTA). Sponsors are also reminded of the importance of timely reporting of SAEs (Serious Adverse Events) and of SUSARs (Suspected Unexpected Serious Adverse Reactions) to the competent authorities, in order to protect the safety of the participants. In addition, we encourage sponsors of COVID-19 trials to post the relevant results as soon as it is feasible, also before the deadline.
14-11-2022: Users have reported issues with uploading CTA XML files on EudraCT. Our IT is currently working on this, we thank you very much for your understanding and patience. Update 21Nov2022: this issue is now solved.
bron: EudraCT public home page
European Medicines Agency (EMA)
Good Clinical Practice (GCP), European Medicines Agency (EMA)
Good Clinical Trials Collaborative
Health Products Regulatory Authority (HPRA) (Ierland)
Health-RI
International Coalition of Medicines Regulatory Authorities (ICMRA)
- Best practices to fight antimicrobial resistance (21-11-2022). EMA.
- ICMRA report on AMR best practices (November 2022)
International Council for Harmonisation (ICH)
Kunstmatige Intelligentie
Methodology, Statistics
- High-dimensional propensity scores for empirical covariate selection in secondary database studies: Planning, implementation, and reporting (08-11-2022). Pharmacoepidemiology and Drug Safety.
- Transparency of high-dimensional propensity score analyses: Guidance for diagnostics and reporting (29-01-2022). Pharmacoepidemiology and Drug Safety.
Nationaal Comité advies dierproevenbeleid (NCad)
- E-nieuws Nationaal Comité advies dierproevenbeleid
- Nieuwe samenstelling NCad
- Uit EARA News: veel te weinig fondsen voor het goedkeuren van diervrije methoden
- Symposium over Next level non-animal testing 2 december
- Biotechnische dagen 7 & 8 december
- Workshops over ongeriefinschatting en verfijning 25 en 26 januari
NHS Health Research Authority (HRA)
- Access all areas? – a blog by Lou Silver, Equality Diversity and Inclusion Manager Health Research Authority (23-11-2022).
- Health Data Research UK joins public involvement pledge (15-11-2022).
- Indemnity cover for NHS staff delivering research (20-10-2022).
Orphan medicinal products, Weesgeneesmiddelen
- Commission Notice Guideline on the format and content of applications for designation as orphan medicinal products and on the transfer of designations from one sponsor to another (2022/C 440/02) (21-11-2022). Official Journal of the European Union.
- Weesgeneesmiddelen, een routekaart voor de toekomst. Vereniging Innovatieve Geneesmiddelen.
Pharma.be
- Farmaceutische bedrijven en patiëntenorganisaties ontmoeten elkaar (21-11-2022).
- Innovatieve geneesmiddelen vergen innovatieve regelgeving (21-11-2022).
QT prolongation, Thorough QT/QTc (TQT) study
Regulatory Science Network Netherlands (RSNN)
Research ethics, Research integrity, Misconduct
- Ook tien jaar na de fraude duikt het sjoemelonderzoek van Diederik Stapel nog steeds op (25-11-2022). De Volkskrant.
- ‘But how will you ensure the objectivity of the researcher?’ Guidelines to address possible misconceptions about the ethical imperatives of community-based research (22-11-2022). Research Ethics.
- UK at a ‘key moment’ for research integrity (22-11-2022). *Research Professional.
- Just how important is the problem of predatory publishing? (21-11-2022). LSE.
- Research integrity guidelines in the academic environment: The context of Brazilian institutions with retracted publications in health and life sciences (28-10-2022). Front. Res. Metr. Anal. 7:991836. doi: 10.3389/frma.2022.991836.
- A Rough Guide to Spotting Bad Science (02-04-2014). Compound Interest.
Retraction Watch Database
Simultaneous National Scientific Advice (SNSA)
- Launch of phase 2 of the simultaneous national scientific advice pilot (22-11-2022). HMA.
- Simultaneous National Scientific Advice (SNSA). HMA.
- Accelerating Clinical Trials in the EU (ACT EU) (22-11-2022). EMA
In November 2022, the EU Innovation Network launched the second phase of the simultaneous national scientific advice (SNSA) pilot. SNSA is intended for situations where an applicant wishes to obtain scientific advice from more than one national competent authority (NCA) at the same time. This is meant to enhance the quality and consistency of such advice. Following endorsement by the Heads of Medicine Agencies (HMA), the second phase of the SNSA pilot will run for a two-year period until the end of 2024. It incorporates an optimised procedure to maximise the benefits for both applicants and competent authorities.
- Guidance for applicants on simultaneous national scientific advice (SNSA) briefing book format and content (22-11-2022).
- Guidance for applicants on simultaneous national scientific advice (SNSA) phase 2 pilot (from October 2022) – Optimised process (22-11-2022).
- Application form to request a simultaneous national sientific advice (SNSA) (22-11-2022).
- Science advice on medicines for Human use in the EU medicines regulatory network (22-11-2022).
- List of NCA’s participating in the simultaneous national scientific advice (SNSA) pilot phase 2 (22-11-2022).
- België – Federaal Agentschap voor Geneesmiddelen en Gezondheidsproducten (FAGG)
US Food and Drug Administration (FDA), HIPAA Privacy and Security Rules, Verenigde Staten
- Enforcement Policy Regarding Investigational New Drug Requirements for Use of Fecal Microbiota for Transplantation to Treat Clostridium difficile Infection Not Responsive to Standard Therapies (25-11-2022). FDA.
- Privacy and Security — Protecting Patients’ Health Information (24-11-2022). NEJM.
Vereniging Innovatieve Geneesmiddelen
- ‘Maak klinisch onderzoek bespreekbaar voor patiënt én samenleving’ (22-11-2022).
- Feiten en frames: op naar een vruchtbare samenwerking tussen academie en industrie (22-11-2022).
- Doorpakken nodig voor ‘Nederland als medicijnhub’ (22-11-2022).
Wet zeggenschap lichaamsmateriaal (Wzl)
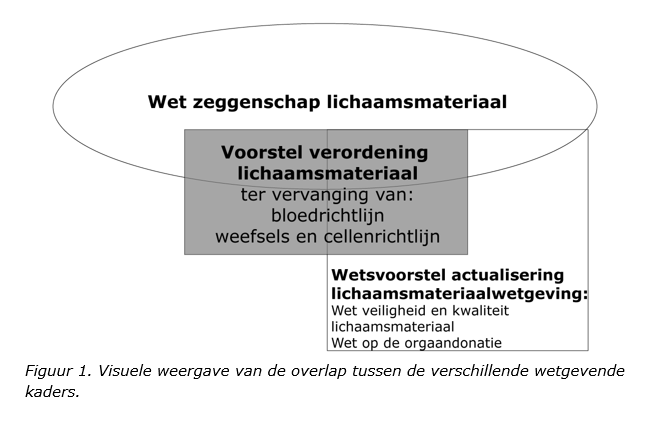
Overgenomen uit: Brief regering d.d. 22-11-2022.
- Brief regering d.d. 06-10-2022, Minister van Binnenlandse Zaken en Koninkrijksrelaties.
- Bijlage bij brief regering: Overzicht aanvullende werking Wzl (06-10-2022).
ZonMw, Hergebruik data, Findable, Accessible, Interoperable and Reusable (FAIR)
- Zijn onze data klaar voor de toekomst? Het FAIR maken van data in projecten over antimicrobiële resistentie en infectieziekten.
- RIVM vaccinatiedata en GGD testdata worden toegankelijk gemaakt voor oversterfte onderzoek via CBS (24-10-2022).
- Aandacht voor gender en sekse in onderzoek blijft nodig (22-10-2022).
Overig
- Weekend reads: What should happen to a paper by Theranos?; Diederik Stapel continues to be cited; a scientist accused of hiding China ties wins $2 million (26-11-2022). Retraction Watch.
- Big Pharma’s Twitter exodus; Merck wagers $1.35B on buyout; $3.5M gene therapy; and more (26-11-2022). Endpoints News.
- Biggest drug companies halted Twitter ad buys after Lilly insulin spoof (21-11-2022). Endpoints News.
- Rathenau: veiligheid biotechnologieonderzoek gewaarborgd, maar overheid moet kennis vergroten (22-11-2022). HollandBIO.
- Science in motion: A qualitative analysis of journalists’ use and perception of preprints. PLOS ONE 17(11): e0277769.
- Mapping the content of comments on bioRxiv and medRxiv preprints (24-11-2022). bioRxiv 2022.11.23.517621.
- External Comparator Groups Derived from Real-world Data Used in Support of Regulatory Decision Making: Use Cases and Challenges. Curr Epidemiol Rep (2022).
- Gene name errors: Lessons not learned. PLOS Computational Biology 17(7): e1008984.
Op de hoogte blijven van actueel nieuws, bijeenkomsten en publieke consultaties