Uitgelicht en gespot op internet (week 38, 2023)
Hierbij een nieuwe uitgave van ‘Uitgelicht en gespot op internet‘, een supplement van de ‘Nieuwsbrief voor Goede Onderzoekspraktijken‘, met een overzicht van nieuws en andere berichten die zijn gespot op het internet.
Vincent Bontrop
www.bontrop.com
U kunt met mij linken via LinkedIn.
EU-X-CT Cross-border Access to Clinical Trials Initiative
Clinical trial results reporting
Data delen, Data structure, Data Management Plans
- Umbrella Data Management Plans to Integrate FAIR Data: Lessons From the ISIDORe and BY-COVID Consortia for Pandemic Preparedness. Data Science Journal, 22(1), p.52.
- Pfizer To Provide Individual Participant Data At Scale (21-09-2023). Clinical Leader.
- Freeing “data” from the Documents they live in (17-09-2023). Futurpositif.
- Feasibility Assessment of Using CDISC Data Standards for In Silico Medical Device Trials”, Journal of the Society for Clinical Data Management 3(3).
Diversiteit, Inclusiviteit, Representativiteit
Embryowet: Voorstel van wet van de leden Paternotte en Hermans tot wijziging van de Embryowet in verband met de afschaffing van het tijdelijk verbod op het doen ontstaan van embryo’s voor wetenschappelijk onderzoek
Op 27 september 2023 zal de procedurevergadering van de vaste commissie voor Volksgezondheid, Welzijn en Sport (VWS) plaatsvinden, klik hier. De voortgang van het Wetsvoorstel ‘Voorstel van wet van de leden Paternotte en Hermans tot wijziging van de Embryowet in verband met de afschaffing van het tijdelijk verbod op het doen ontstaan van embryo’s voor wetenschappelijk onderzoek‘ is te volgen via de website van de Tweede Kamer, klik hier. |
Ethiek
- A Top New York Hospital, an Unapproved Treatment and an F.D.A. Warning (20-09-2023). The New York Times.
- A parent’s dilemma: what if our son asks ‘why did you try to change me?’ (16-09-2023). The Guardian.
European Medicines Agency (EMA), Inspectors Working Group, GCP, Inspection procedures and guidance, Phase I units, biological medicinal products
- Annex III to procedure for conducting good-clinical-practice inspections requested by the EMA: Computer systems (updated 22-09-2023)
- Annex V to procedure for conducting GCP inspections requested by the EMEA: Phase I units (updated 22-09-2023).
- Questions and answers for biological medicinal products (updated 18-09-2023)
- Clinical Trials Highlights newsletter 15 (juli 2023). EMA.
Fraud, Misconduct, Integrity, Pseudoscience
- Vaccine specialist Peter Hotez: scientists are ‘under attack for someone else’s political gain’ (21-09-2023). Nature.
- Guest post: Genomics has a spreadsheet problem (20-09-2023). Retraction Watch.
- What facilitates or prevents academic fraud in a Colombian faculty of medicine–Protocol of a study using fuzzy cognitive mapping. PLOS ONE 18(9): e0291737.
- Australia needs a science fraud watchdog — one with teeth (20-09-2023). Crikey.
- How to curb bias in manuscript assessments. Nat. Biomed. Eng 7, 1055–1056 (2023).
- Consciousness theory slammed as ‘pseudoscience’ — sparking uproar (20-09-2023). Nature.
- It’s time to talk about research culture and the REF (14-09-2023). Wonkhe.
- The Publication Facts Label: Ascertaining a Publication’s Adherence to Scholarly Standards (12-09-2023). In SciELO Preprints.
- Defining “recklessness” in research misconduct proceedings. Account Res. 2023 Sep 11:1-23. doi: 10.1080/08989621.2023.2256650. Epub ahead of print. PMID: 37694962.
German Biobank Node
- Gesundheitsdatennutzungsgesetz im Überblick (12-09-2023).
- Starterkit für Biobanken im Aufbau (20-09-2023).
- Material Transfer Agreement: GBN entwickelt Vorlage (08-09-2023).
Global Alliance for Genomics and Health (GA4GH)
- Supporting a collaborative and safe GA4GH: introducing the Code of Ethics and Community Conduct (15-09-2023).
- GA4GH Code of Ethics and Community Conduct (CECC).
Inspectie Gezondheidszorg en Jeugd (IGJ)
International Society for Stem Cell Research (ISSCR)
Kunstmatige Intelligentie (KI)
League of European Research Universities (LERU)
- A toolbox is not enough (20-09-2023). LERU.
- New initiatives to empower research careers and to strengthen the European Research Area (13-07-2023). European Commission.
Medicines and Healthcare products Regulatory Agency (MHRA)
- Collection: Medicines, medical devices and blood regulation and safety: Clinical trials and investigations (21-09-2023).
- Collection: Medicines, medical devices and blood regulation and safety: Good practice, inspections and enforcement (21-09-2023).
- Guidance: The Innovative Devices Access Pathway (IDAP) (19-09-2023).
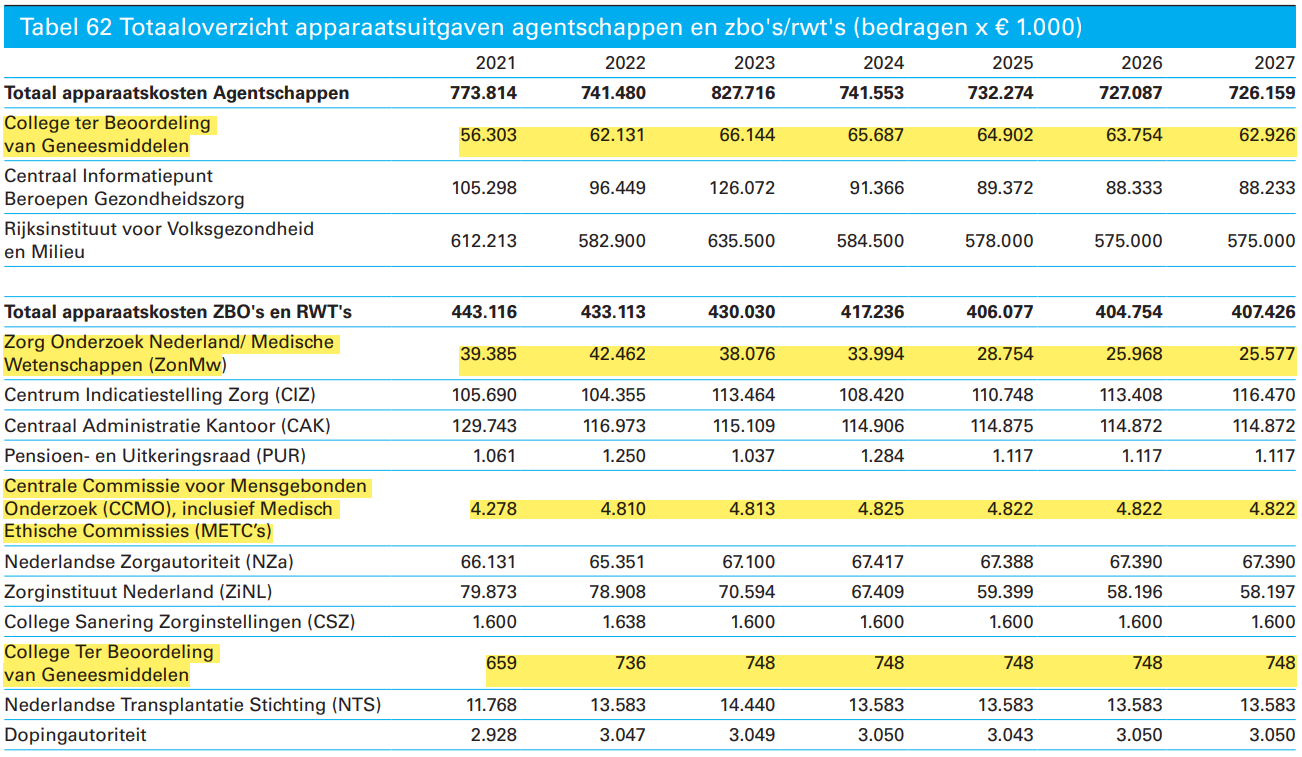
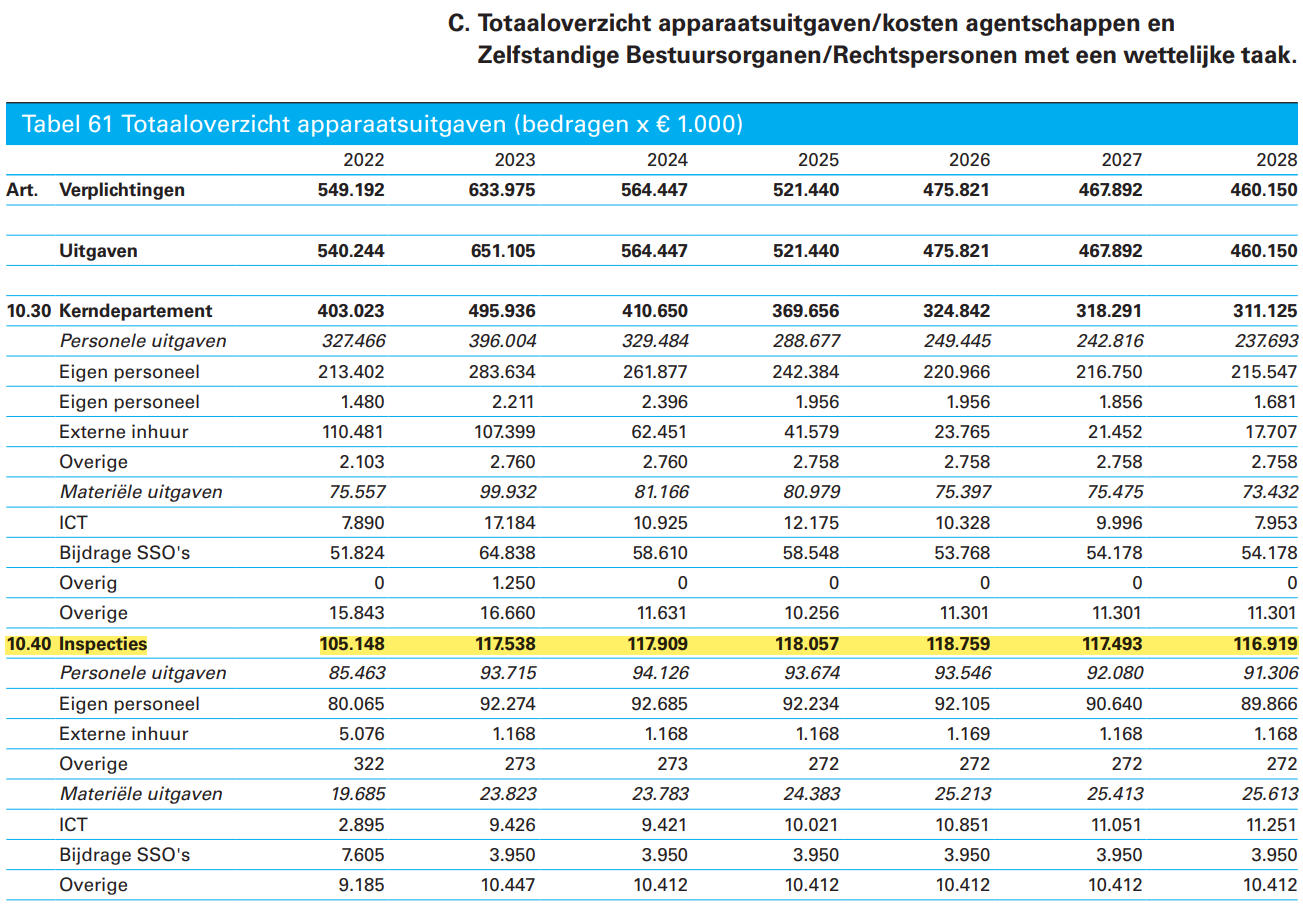
Ministerie van Volksgezondheid, Welzijn en Sport (VWS)
|
Nationaal Comité advies dierproevenbeleid (NCad)
- Zienswijze Afwegingskader voor het Prioriteren van Dierproeven voor Vervanging (09-08-2023).
- 6e Harry Blomberaad Voorkomen van (ernstig) ongerief goed bezocht (06-08-2023).
- E-nieuws Nationaal Comité advies dierproevenbeleid (september 2023).
Nieuwsbrief voor Goede Onderzoekspraktijken
Nederlandse Federatie van Universitair Medische Centra (NFU)
Participatiekompas
- 3 sterke proefschriften over patiëntenparticipatie
- Effective and sustainable patient participation – The patients’ perspective in CF care and CF research (2023)
- Proefschrift & podcast – Working Together, Learning Together: Towards Universal Design for Research (2021)
- Towards meaningful and sustainable patient involvement in health research decision-making – (2021)
- Mensen met een verstandelijke beperking adviseren onderzoekers.
- Collectie patiëntervaringsverhalen beschikbaar voor systematisch onderzoek.
Patient Engagement Synapse
- How Can Medical Device Manufacturers Make Best Use Of Patient Preference Information? (30-08-2023).
- Characterising meaningful patient and public involvement in the pharmaceutical industry research setting: a retrospective quality assessment (23-08-2023).
- The global Patient Engagement map and network
Universiteiten van Nederland
US Food and Drug Administration’s (FDA), National Institutes of Health (NIH)
- Make Your Voice Heard on NIH’s Draft Scientific Integrity Policy (22-09-2023).
- Draft guidance: Considerations for the Conduct of Clinical Trials of Medical Products During Major Disruptions Due to Disasters and Public Health Emergencies (21-09-2023).
- FDA issues draft guidance regarding confirmatory evidence of clinical trials (18-09-2023).
- FDA will continue to use remote inspection tools to assess pending applications (22-09-2023). Regulatory Focus.
- FDA guidance on conducting trials during emergencies shifts away from COVID-19 (21-09-2023). Regulatory Focus.
- FDA expands types of acceptable confirmatory evidence in new guidance (19-09-2023). Regulatory Focus.
- FDA weight-loss device guidances address clinical and non-clinical studies (18-09-2023). Regulatory Focus.
- Logistics Unwrapped: A Roadmap to Successful FDA Meetings in In-Person and Hybrid Formats (18-09-2023). FDA Law Blog.
Overig
- Weekend reads: Who should pay for sleuthing?; the Gino retraction requests; university ‘halts projects over fraud investigation’ (23-09-2023). Retraction Watch.
- Human embryo models: the importance of national policy and governance review. Curr Opin Genet Dev. 2023 Oct;82:102103. doi: 10.1016/j.gde.2023.102103. Epub 2023 Aug 22. PMID: 37619506.
- Collaborating with regulatory authorities to implement our innovative methodologies (20-09-2023). Oncode Accelerator.
- Super-precise CRISPR tool enters US clinical trials for the first time (18-09-2023). Nature.
- ELRIG sets up science engagement group for biotechs, CROs and academics (18-09-2023). Clinical Insider.
- First global survey reveals who is doing ‘gain of function’ research on pathogens and why (15-09-2023). Nature.
- The unblinding of statisticians in clinical trials: commentary on Iflaifel et al., Trials 2023. Trials 24, 579 (2023).
- Johns Hopkins Medicine commits to Trial Innovation Center after securing $24.5m (11-09-2023). Clinical Insider.
Op de hoogte blijven van actueel nieuws, bijeenkomsten en publieke consultaties