Uitgelicht en gespot op internet (week 39, 40 & 41, 2023)
Hierbij een nieuwe uitgave van ‘Uitgelicht en gespot op internet‘, een supplement van de ‘Nieuwsbrief voor Goede Onderzoekspraktijken‘, met een overzicht van nieuws en andere berichten die zijn gespot op het internet.
Mede door andere werkzaamheden is het mij niet gelukt de voorgaande weken een nieuwe ‘Uitgelicht en gespot’ op mijn weblog te plaatsen. Inmiddels heb ik mijn e-mailboxen doorgeploegd, mijn LinkedIn bookmarks nagelopen en w.v.t.t.k. bijeengebracht en heb ik dit bomvolle overzicht weten te produceren. De afgelopen dagen heb ik wel meerdere malen moeten denken aan de quote uit Flodder, “Leuk zo’n feestje, maar ‘t geeft altijd zo’n rommel hé?!“. Maar uiteindelijk is het ook deze keer weer gelukt. Mocht er nog wat nieuws ergens zijn blijven liggen of vergeten zijn, geef het gerust aan mij door via het contactformulier.
Veel leesplezier!
Vincent Bontrop
www.bontrop.com
U kunt met mij linken via LinkedIn.
Centrale Commissie Mensgebonden Onderzoek (CCMO)
Centre for Future Affordable Sustainable Therapy development (FAST)
- Paul Smits: ‘Het ideale vehikel om ambities te verzilveren’ (02-10-2023).
- ‘We kunnen nieuwe producten sneller en efficiënter ontwikkelen’ (28-09-2023).
- Uitgelicht traject: Haalbaarheidsstudie door TNO: EMA data analyse (27-09-2023).
- Start van programma regulatoire pandemische paraatheid (08-08-2023).
| Clinical Trials Regulation (CTR)
Accelerating Clinical Trials in the EU (ACT-EU)
Clinical Trials Expert Group (CTEG) EudraLex De wijzigingen ten opzichte van de voorgaande versie zijn:
European Medicines Agency (EMA), Management Board, Transparancy, Clincal trial data publication
Federaal Agentschap voor Geneesmiddelen en Gezondheidsproducten (FAGG) (België) Medical Device Coordination Group (MDCG) Overige berichtgeving |
Clinical trials, ECRAID, Good Clinical Trials Collaborative, Decemtralised clinical trials
- Whitepaper: Navigating the regulatory labyrinth of technology in decentralised clinical trials. Keeping up to date and implementing the latest guidelines. ICON.
Geintresseerden die het document willen lezen maar liever anoniem willen blijven weten mij te vinden 😉 - Do the Outcomes of Clinical Efficacy Trials Matter in Regulatory Decision-Making for Biosimilars? (13-10-2023). BioDrugs.
- What is the Database lock in Clinical Trials? (10-10-2023). GCP-Mindset, via YouTube.
- Enabling “Good” Clinical Research In LMICs With The Good Clinical Trials Collaborative (27-09-2023). Clinical Leader.
- Clinical trial platforms for new and neglected antimicrobials (22-09-2023). Global Antibiotic Research & Development Partnership (GARDP), via YouTube.
Data delen, Data hergebruik, Data Management Plans, Data beveiling, Privacy
- Safe Societal Sharing of Data: a Multiparty Computing Solution (October 2023). Teus Kappen & Marc Padros Goossens.
- Vergelijking van wet-en regelgeving in Europa voor hergebruik van gezondheidsdata voor onderzoek en innovatie (25-08-2023). Health-RI.
- 23andMe User Data Stolen in Targeted Attack on Ashkenazi Jews (06-10-2023). WIRED.
Dutch Clinical Research Foundation (DCRF)
ELIXIR
European Health Data Space (EHDS)
European Medicines Agency (EMA), International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH)
- Updated document – ICH Q9 Quality Risk Management (13-10-2023).
ICH Q9 Quality Risk Management has been updated and the current version is effective from 26/07/2023 It provides principles and examples of tools for quality risk management that can be applied to different aspects of pharmaceutical quality. These aspects include development, manufacturing, distribution, and the inspection and submission/review processes throughout the lifecycle of drug substances, drug products, biological and biotechnological products. - Queries Raised During Oncology Business Pipeline Meetings at the European Medicines Agency: A 5-Year Retrospective Analysis (04-08-2023). Clinical Pharmacology & Therapeutics.
Europese Unie (EU), Europese Commissie, Geneesmiddelenwetgeving, Wetgeving, Regeldruk
- Briefing ‘Revision of the EU pharmaceutical legislation’ (september 2023).
- Watch this space: launch of EMA-HMA catalogues of data sources and non-interventional studies in early 2024 (27-09-2023).
The European Medicines Regulatory Network is developing two new public catalogues, one for real-world data sources and one for non-interventional studies. These catalogues will replace and enhance the current ENCePP Resources Database and the EU PAS Register at the beginning of next year.
Fraud, Misconduct, Integrity, Pseudoscience
“… anyone could reflect on the matters of research ethics and research integrity, and anonymously share their reflections with the BEYOND team.
The public consultation provides a brief online response form where we aim to explore research ethics and research integrity practice and research misconduct from the point of view of the general public and different stakeholders. The public consultation is central to BEYOND project for building a dialogue with the society and stakeholders on research ethics and research integrity. We truly appreciate every submission, as it brings us one step closer towards our goal of preventing research misconduct.”
bron: BEYOND nieuwsbericht
- Knowledge, attitudes and practices about research misconduct among medical residents in Southwest China: a cross-sectional study (10-10-2023). Research Square.
- To guard against fraud, medical research should be a profession: A book excerpt (04-10-2023). Retraction Watch.
- AI beats human sleuth at finding problematic images in research papers (03-10-2023). Nature.
- How to modernize medical evidence for the misinformation era. Nat Med (2023).
- Maternal health points to need for oversight on scientific research (26-09-2023). COSMOS.
- Wanneer is iets wetenschappelijk bewezen? Hoe het grijze gebied van wetenschappelijke verificatie wordt misbruikt. Sjamadriaan.
- Editiorials: An Overdue Due Process for Research Misconduct (25-09-2023). The Harvard Crimson.
- The Band of Debunkers Busting Bad Scientists. Stanford’s president and a high-profile physicist are among those taken down by a growing wave of volunteers who expose faulty or fraudulent research papers (24-09-2023). WSJ.
- A Quantitative Study of Inappropriate Image Duplication in the Journal Toxicology Reports (10-09-2023). bioRxiv.
Gentherapie en GGO
- Gene therapies for rare diseases are under threat. Scientists hope to save them (06-10-2023). Nature.
- Gene therapy death was caused by an unknown risk of the virus used, study suggests (27-09-2023). STAT.
- The next-generation CAR-T therapy landscape (06-09-2023). Nature Reviews Drug Disco very.
Health Holland
Health-RI
- Health-RI Architectuurontwerp Versie v1.0. Health-RI.
- Terugblik webinar V1.0 – Save the date: open consultatie architectuur V2.0 (15-06-2023). Health-RI.
- Nationale initiatieven voor een gezondheidsdata-infrastructuur uit Duitsland, Zwitserland en Nederland ontmoeten elkaar in Berlijn, juni 2023 (06-07-2023). Health-RI.
- Health-RI belangrijke partner in Genomic Data Infrastructuur project (27-06-2023). Health-RI.
Informed consent, Geinformeerde toestemming, eConsent, Electronische toestemming
- eConsent: praktische aanvulling op CCMO handreiking (13-10-2023). DCRF.
- Academic and Private Partnership to Improve Informed Consent Forms Using a Data Driven Approach (25-09-2023). The American Journal of Bioethics: Vol 0, No 0.
- Blog: Improving information for research participants (27-09-2023). Health Research Authority.
- Evaluation of CTRL: a web application for dynamic consent and engagement with individuals involved in a cardiovascular genetic disorders cohort (14-09-2023). Eur J Hum Genet (2023).
- The reuse of genetic information in research and informed consent (13-09-2023). Eur J Hum Genet (2023).
- Opt-out of opt-in? Zo zwart wit is het niet! (12-09-2023). Health-RI.
- Comparative Effectiveness of eConsent: Systematic Review. J Med Internet Res 2023;25:e43883.
Inspectie Gezondheidszorg en Jeugd (IGJ)
- Eerste bezoeken laten zien dat in ziekenhuizen te weinig aandacht is voor controle op financiële relaties tussen artsen en leveranciers van medische hulpmiddelen (21-09-2023).
- Hoe beoordeel je als farmaceutisch bedrijf een sponsorverzoek? Vijf tips (september 2023). Marloes Meddens-Bakker via LinkedIn.
Kunstmatige Intelligentie (KI)
- AI’s potential to accelerate drug discovery needs a reality check (10-10-2023). Nature.
- Where Medical Statistics Meets Artificial Intelligence (28-09-2023). NEJM.
Medicines and Healthcare products Regulatory Agency (MHRA), NHS Health Research Authority (HRA)
- New streamlined notification scheme for lowest-risk clinical trials marks start of MHRA overhaul of regulation (12-10-2023).
- Clinical Trials Performance Metrics: October 2022 -September 2023: Assessment of Clinical Trial Authorisation Applications and Substantial Amendments (13-10-2023).
- Guidance: Clinical trials for medicines: apply for authorisation in the UK (updated 12-10-2023).
- Blog: Patient centricity and the UK’s pivotal role in human clinical research (28-09-2023). Health Research Authority.
- The role of genetics in medicine safety (02-10-2023). MedRegs.
- Roundtable event to discuss issues of capacity whilst taking part in long-term research (22-09-2023). Health Research Authority.
- Blog: Why diversity is important in good research (05-09-2023). Health Research Authority.
- programma regulatoire pandemische paraatheid (08-08-2023).
| Medical Device Regulation (MDR)
Medical Device Coordination Group (MDCG)
Medical Device Software |
Medische hulpmiddelen, Neuralink
- Mitigating skin tone bias in linear array in vivo photoacoustic imaging with short-lag spatial coherence beamforming (oktober 2023). Photoacoustics.
- Medical imaging fails dark skin. Researchers fixed it. (10-10-2023). Hub.
- Musk’s Neuralink Seeks Volunteers for Brain Chip Implant Study (20-09-2023). Gizmodo.
- How Neuralink Keeps Dead Monkey Photos Secret (04-10-2023). WIRED.
Nieuwsbrief voor Goede Onderzoekspraktijken
- Nieuwsbrief voor Goede Onderzoekspraktijken nr. 180 (11-10-2023).
- Nieuwsbrief voor Goede Onderzoekspraktijken nr. 179 (02-10-2023).
Nederlandse Vereniging voor Farmaceutische Geneeskunde (NVFG)
Reporting guidelines, Research output
- ACCORD (ACcurate COnsensus Reporting Document): A reporting guideline for consensus methods in biomedicine developed via a modified Delphi (24-08-2023). medRxiv.
- How ChatGPT and other AI tools could disrupt scientific publishing (10-10-2023). Nature.
- Editors’ statement on the responsible use of generative artificial intelligence technologies in scholarly journal publishing (01-10-2023). Bioethics.
- Ending Human-Dependent Peer Review (29-09-2023). The Scholary Kitchen.
- Should research results be published during Ph.D. studies? (23-09-2023). The Hindu.
- A Critical Examination of the Ethics of AI-Mediated Peer Review (02-09-2023). arXiv.
- Presentation and publication skills: Publication governance and pitfalls to avoid (13-06-2023). Clinical Nutrition ESPEN.
Ruimtevaart, Human research in commercial spaceflight
- Global Team Recommends Ethical Framework for Human Research in Commercial Spaceflight (28-09-2023). The Hastings Center.
- Ethically cleared to launch? (28-09-2023). Science.
Podcast Focus over proefpersonen
- Onderstaande podcasts bijelkaar in 1 playlist, klik hier om naar Spotify te gaan.
- Proefpersonen #1 – Hoe is het om mee te doen aan geneesmiddelenonderzoek (06-09-2023). Focus, via Spotify.
- Proefpersonen #2 – In alle vormen, maten en kleuren graag (13-09-2023). Focus, via Spotify.
- Proefpersonen #3 – Gezond oud worden dankzij honderdduizenden vrijwilligers (20-09-2023). Focus, via Spotify.
- Proefpersonen #4 – Wat de wetenschap met jouw data doet (27-09-2023). Focus, via Spotify.
Reproduceerbaarheid
Stichting Code Geneesmiddelen Reclame (CGR)
TransCelerate Biopharma Inc.
- Webinar recording: Understanding Case Reporting Transmission Networks in Drug Safety: The Impact of Replication (18-09-2023). Via YouTube.
- Slides: Understanding Case Reporting Transmission Networks in Drug Safety: The Impact of Replication (08-09-2023).
- Intelligent Automation Opportunities Pharmacovigilance.
US Food and Drug Administration’s (FDA), HHS Office of Research Integrity (ORI)
- Draft guidance: Quality Considerations for Topical Ophthalmic Drug Products (13-10-2023).
- Finalc guidance: Investigational COVID-19 Convalescent Plasma: Guidance for Industry (13-10-2023)
- Final guidance: Data Standards Catalog (12-10-2023).
- Draft guidance: Stimulant Use Disorders: Developing Drugs for Treatment (05-10-2023).
- Draft guidance: Graft-versus-Host Diseases: Developing Drugs, Biological Products, and Certain Devices for Prevention or Treatment (28-09-2023).
- FDA Establishes New Advisory Committee on Digital Health Technologies (12-10-2023). FDA.
- Public Health Service Policies on Research Misconduct. A Proposed Rule by the Health and Human Services Department on 10/06/2023.
- Make Your Voice Heard on NIH’s Draft Scientific Integrity Policy (22-09-2023). NIH.
- FDA Moves Beyond COVID-19, But Impacts on COVID-19 Era Clinical Trials Remain (12-10-2023). The FDA Law Blog.
- Diversity plan draft guidance on track before year end, FDA official says (12-10-2023). Regulatory Focus.
- Convergence: Psychedelic drug development is ‘hot,’ but proceed with caution (04-10-2023). Regulatory Foucs.
- Feasibility of Emulating Clinical Trials Supporting US FDA Supplemental Indication Approvals of Drugs and Biologics. JAMA Intern Med. Published online October 02, 2023. doi:10.1001/jamainternmed.2023.4073.
- FDA official discusses use of RWE in cancer research and approvals (29-09-2023). Regulatory Focus.
- CBER revises internal procedures for processing clinical holds, NDAs and BLAs (28-09-2023). Regulatory Focus.
Xenotransplantatie, Genoombewerking
- A monkey survived two years with a miniature pig’s kidney (11-10-2023). Science News.
- Myopic View of Xenotransplantation (25-09-2023). The Hastings Center.
- Genetically Modified Pig’s Heart Is Transplanted Into a Second Patient (22-09-2023). The New York Times.
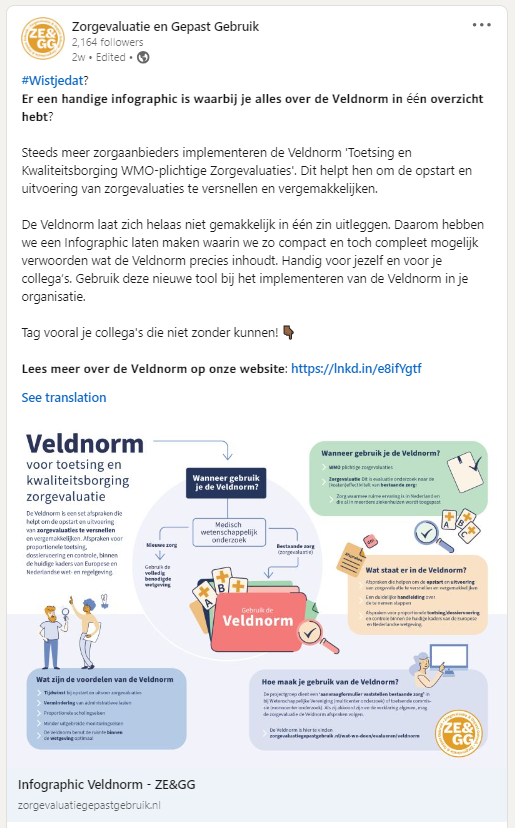
Zorgevaluatie en Gepast Gebruik (ZE&GG)
- Weekend reads: ‘Egregious misconduct’ by biotech collaborator; an IVF doctor with allegedly fake credentials; ChatGPT not the problem in publishing (14-10-2023). Retraction Watch.
- Weekend reads: ‘The band of debunkers’; a superconductor retraction request; ‘the banality of bad-faith science’ (30-09-2023). Retraction Watch.
- Grote opkomst onder jongeren voor onderzoek gezondheid (10-10-2023). Skipr.
- How a Big Pharma Company Stalled a Potentially Lifesaving Vaccine in Pursuit of Bigger Profits (04-10-2023). ProPublica.
- Community-based management of a five-arm randomised clinical trial in COVID-19 outpatients in South Africa: challenges and opportunities (04-10-2023). Trials.
- Navigating the risks and benefits of AI: Lessons from nanotechnology on ensuring emerging technologies are safe as well as successful (02-10-2023). The Conversation.
- Ethics of Adaptive Designs for Randomized Controlled Trials (01-10-2023). Ethics & Human Research.
- Randomized Clinical Trial Visual Abstract Display and Social Media–Driven Website Traffic (29-09-2023). JAMA.
- Complicated regulatory decision-making following inconsistent trial results: the issue with ibrutinib for mantle cell lymphoma (21-09-2023). Nat Rev Clin Oncol.
- LUMC en CHDR verstevigen samenwerking met opening klinische research unit (19-09-2023). LUMC.
- IQVIA and three leading university medical centers launch the first Prime Site in the Netherlands to advance clinical and real-world research (11-09-2023). IQVIA.
- A novel approach to boost drug development in paediatric oncology (22-08-2023). Nature Reviews Drug Discovery.
- Investigating the origins of recent pharmaceutical innovation (05-07-2023). Nature.
Op de hoogte blijven van actueel nieuws, bijeenkomsten en publieke consultaties