Uitgelicht en gespot op internet (week 17, 2023)
Hierbij een nieuwe uitgave van ‘Uitgelicht en gespot op internet‘, een supplement van de ‘Nieuwsbrief voor Goede Onderzoekspraktijken‘, met een overzicht van nieuws en andere berichten die zijn gespot op het internet.
Vincent Bontrop
www.bontrop.com
U kunt met mij linken via LinkedIn.
Belgian Association of Research Ethics Committees (BAREC)
- Statement on decentralized trials (18-04-2023).
- Medical Device Regulation (MDR): role local EC + timelines EC (18-04-2023).
Clinical Trials Coordination Group (CTCG)
Clinical Trial Regulation (CTR), Clinical Trials Information System (CTIS)
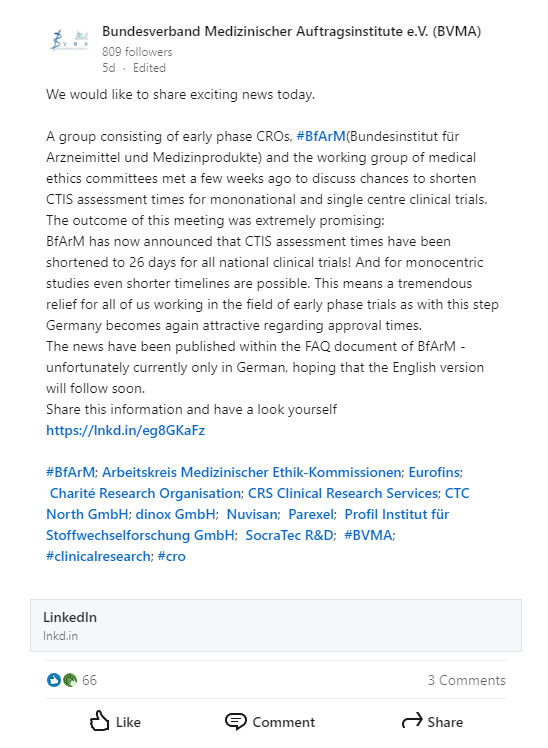
Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM) |
Clinical trials
- Non-traditional sites a path to more diverse clinical trials, say researchers (25-04-2023). Clinical Insider.
- Q&A: Improving paediatric clinical trials with the language of play (25-04-2024). Clinical Trials Arena.
- Effect of the Data Collection Method on Mobile Phone Survey Participation in Bangladesh and Tanzania: Secondary Analyses of a Randomized Crossover Trial. JMIR Form Res 2023;7:e38774.
- Statistical analyses of ordinal outcomes in randomised controlled trials: protocol for a scoping review. Trials 24, 286 (2023).
- RCT-DUPLICATE Initiative. Emulation of Randomized Clinical Trials With Nonrandomized Database Analyses: Results of 32 Clinical Trials. JAMA. 2023;329(16):1376–1385. doi:10.1001/jama.2023.4221
Council for International Organizations of Medical Sciences (CIOMS)
” … The objective of the meeting was to present the results of a recently published scoping review on the considerations of sex and gender dimensions by research ethics committees and to initiate a discussion on the role of research ethics committees (RECs) and research ethics guidelines in addressing the gender bias in research.”
Dierexperimenteel onderzoek
- 5 misvattingen over proefdieronderzoek (24-04-2023). Dash of Ginger.
- Norecopa newsletter 2-2023 (17 April 2023).
EQUATOR network (Enhancing the QUAlity and Transparency Of health Research)
- EQUATOR Network Newsletter April 2023 (27-04-2023).
- Sharing opportunities for patients to influence methodology research
- The EQUATOR Network reporting guideline database audit has been published
- Biomedical research data sharing and management: The Ottawa Data Champions project
- UK EQUATOR Centre contributes to the HRA Make It Public week
- Journals update guidelines after review highlights incomplete reporting of interventions
- Contacting trial authors may improve the often poor description of interventions in COVID studies
- Poor methodology and reporting of prediction models developed using machine learning in oncology
- Guidance for reporting studies using artificial intelligence natural language models is now under development
European Medicines Agency (EMA)
“EMA intends to gradually resume clinical data publication from September 2023, having temporarily suspended this activity for all products except Treatments and vaccines for COVID-19.”
bron: EMA website ‘Support for industry on clinical data publication’
EU’s pharmaceutical legislation
- Reform of the EU pharmaceutical legislation
- A new pharmaceutical legislation for the world of tomorrow (27-04-2023).
- Questions and Answers on the pharmaceutical legislation
- European Health Union: Commission proposes pharmaceuticals reform for more accessible, affordable and innovative medicines (26-04-2023).
- EU releases draft legislation that will reshape pharma regulation (26-04-2023). Regulatory Focus.
- Proposed Amendments to the EU Regulatory Framework for Medicinal Products (26-04-2023). Arnold & Porter, BioSlice Blog.
- EU pharma-legislation risks sabotaging Europe’s life science industry putting European patients further away from the cutting-edge of healthcare (26-04-2023). EFPIA.
- Rem op ontwikkeling nieuwe geneesmiddelen in Europa (26-04-2023). Vereniging Innovatieve Geneesmiddelen.
- Nieuwe Europese wetgeving voor geneesmiddelen (25-04-2023). Vereniging Innovatieve Geneesmiddelen.
- ‘A tug of war’: Europe braces for new legislation with far-reaching impacts on pharma and patients (24-04-2023). STAT.
Global Alliance for Genomics and Health (GA4HA)
- GA4GH GDPR Brief: GDPR going forward: prospects of resuming transatlantic data sharing (27-04-2023).
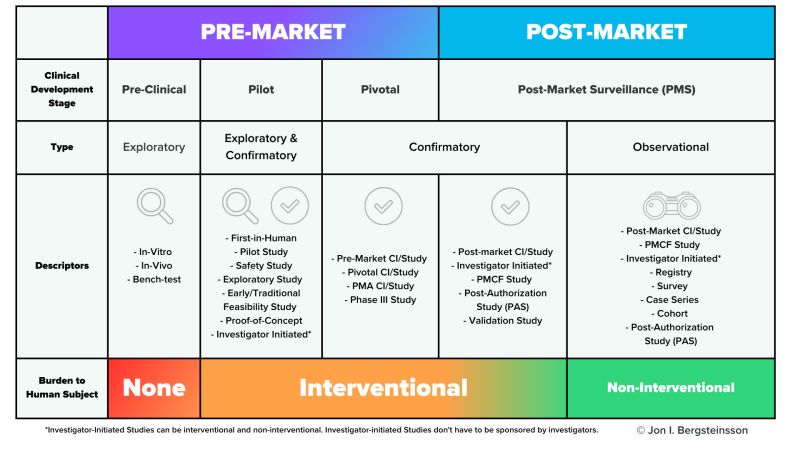
Medische hulpmiddelen, Clinical investigation, clinical study, or a clinical trial
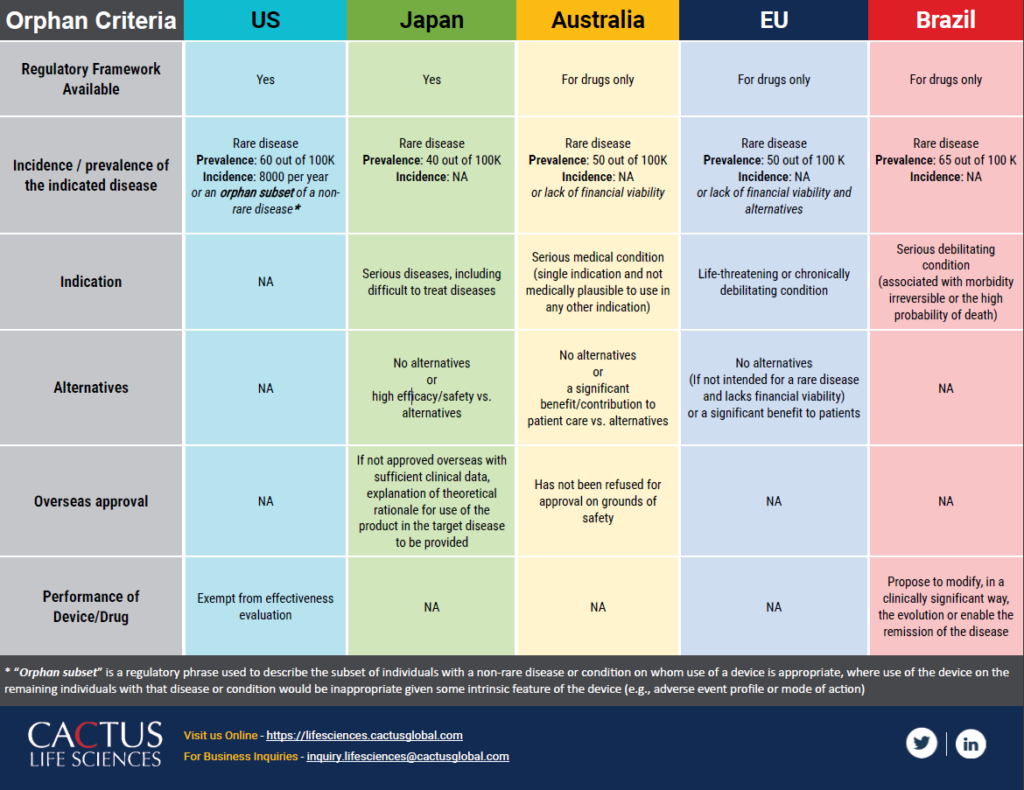
Medische hulpmiddelen, Orphan Drug/Device Criteria across the US, Japan, Australia, EU and Brazil markets
Nieuwsbrief voor Goede Onderzoekspraktijken
Nivel
- Toestemming vragen aan patiënten voor hergebruik zorgdata kan leiden tot vertekende resultaten in onderzoek (28-04-2023).
- Opt-in and opt-out consent procedures for the reuse of routinely recorded health data in scientific research and their consequences for consent rate and consent bias: systematic review. Journal of Medical Internet Research: 2023, 25, Art. nr. e42131.
Participatiekompas
- Aandacht voor begroten van patiëntenparticipatie in projecten (26-04-2023).
- Onderzoek naar betrokkenheid ervaringsdeskundigen bij gezondheidsfondsen (26-04-2023).
- Patiëntenparticipatie bij METC-aanvraag (16-03-2023).
Stichting Code Geneesmiddelen Reclame (CGR)
US Food and Drug Administration’s (FDA)
- S12 Nonclinical biodistribution considerations for gene therapy products: Guidance for Industry (01-05-2023).
- FDA’s Draft Guidance on Externally Controlled Trials Answers Some Questions, Leaves Others Unanswered (24-04-2023). Hyman, Phelps & McNamara PC, FDA Law Blog.
Overig
- Weekend reads: ‘No gender bias in academic science;’ an editor is fired; foreign research fraud in Australia (29-04-2023). Retraction Watch.
- Trial activity, R&D spending soar in 2022, but diversity issues remain (25-04-2023). Clinical Insider.
- How Do Scientists Perceive the Relationship Between Ethics and Science? A Pilot Study of Scientists’ Appeals to Values. Sci Eng Ethics 29, 15 (2023).
- Retracted publications in autism research are mostly concerned with ethical misconduct (19 April 2023). Health Information & Libraries Journal.
- Say what? The principal investigators who pass down wisdom through humour (24-04-2023). Nature.
- What is a digital patient twin? (21-03-2023). Siemens Healthineers.
Op de hoogte blijven van actueel nieuws, bijeenkomsten en publieke consultaties