Uitgelicht en gespot op internet (week 11, 2023)
Hierbij een nieuwe uitgave van ‘Uitgelicht en gespot op internet‘, een supplement van de ‘Nieuwsbrief voor Goede Onderzoekspraktijken‘, met een overzicht van nieuws en andere berichten die zijn gespot op het internet.
Vincent Bontrop
www.bontrop.com
U kunt met mij linken via LinkedIn.
Centrale Commissie Dierproeven (CCD), Dierexperimenteel onderzoek
- Jaarverslag CCD 2022 gepubliceerd (14-03-2023).
- Verslag gebruikersplatform 25 november 2022 (13-03-2023).
Centrale Commissie Mensgebonden Onderzoek (CCMO)
Clinical Trials Coordination Group (CTCG)
European Medicines Agency (EMA)
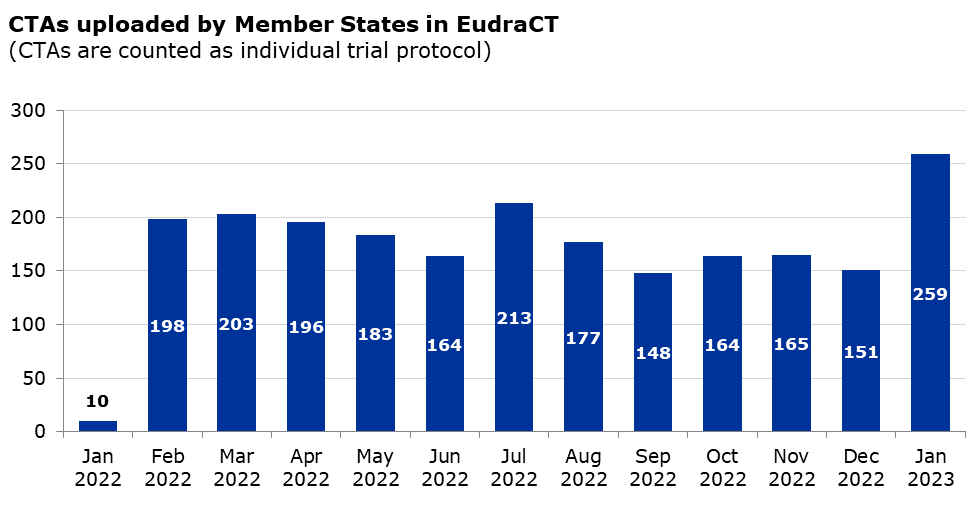
Clinical Trial Regulation (CTR), Clinical Trials Information System (CTIS) |
European CRO Federation (EUCROF)
- EUCROF raises awareness about cross-border trials initiative (13-03-2023). Clinical Insider.
- Trials industry needs to settle on terminology for decentralized studies, says report (13-03-2023). Clinical Insider.
- Decentralised, patient-centric, site-less, virtual, and digital clinical trials? From confusion to consensus (april 2023). Drug Discovery Today.
European Federation of Pharmaceutical Industries and Associations (EFPIA)
Federatie Medisch Specialisten (FMS)
Geneesmiddelen
Genetic studies, Diversity
- National Academies calls for transforming use of racial and ethnic labels in genetics research (14-03-2023). STAT.
- Why experts recommend ditching racial labels in genetic studies (14-03-2023). Science News.
International Council for Harmonisation (ICH)
Integraal Kankercentrum Nederland (IKNL)
International Federation of Associations of Pharmaceutical Physicians and Pharmaceutical Medicine (IFAPP)
Kunstmatige Intelligentie (KI)
Nationaal Comité advies dierproevenbeleid (NCad)
Nieuwsbrief voor Goede Onderzoekspraktijken
Minister van Volksgezondheid, Welzijn en Sport
- Brief regering: Reactie op verzoek commissie over informatie rondom oversterfte (13-03-2023).
- Kuipers: strenger toezicht op beïnvloeding in zorg (13-03-2023). Skipr.
Patiëntenparticipatie
US Food and Drug Administration’s (FDA)
- Draft guidance: Electronic Systems, Electronic Records, and Electronic Signatures in Clinical Investigations: Questions and Answers (15-03-2023).
- Draft guidance: Pharmacogenomic Data Submissions (17-03-2023).
- Draft guidance: Development of Local Anesthetic Drug Products With Prolonged Duration of Effect (15-03-2023).
- Draft Guidance for Industry: Frequently Asked Questions About Medical Foods – Third Edition (15-03-2023).
- Pharma wants FDA’s IND reporting rule better aligned with ICH E2F (15-03-2023). Regulatory Focus.
- EMA-FDA parallel scientific advice program has ‘limited’ uptake (14-03-2023). Regulatory Focus.
- FDA meeting highlights patient recruitment challenges on Rare Disease Day (28-02-2023). Clinical Trials Arena.
- Thousands of Sites, Less Than 100 Investigators: New Authorities Help FDA Maintain Oversight of Clinical Research (27-02-2023).
Overig
- Weekend reads: ‘Strife at eLife’; fraudulent science at Monsanto; cats and chatbots writing papers (18-02-2023). Retraction Watch.
- When journals don’t meet their ethical guidelines, will anyone hold them accountable? (17-03-2023). Retraction Watch.
- ‘Zwarte lijst voor zorgmedewerkers wordt nauwelijks gecheckt’ (11-03-2023). Skipr.
- Electronic health record-based prediction models for in-hospital adverse drug event diagnosis or prognosis: a systematic review, Journal of the American Medical Informatics Association, 2023;, ocad014.
- How NLP Surfaces Adverse Events and Safety Insights to Improve Drug Safety Processes (17-03-2023). Applied Clinical Trials.
- Advancing ICU Care with eConsent (16-03-2023). Applied Clinical Trials.
- Right-Size Your eTMF (16-03-2023). Applied Clinical Trials.
- Connecting real-world data and domain expertise to enhance trial design and planning (16-03-2023). Applied Clinical Trials.
- Petition adds pressure on FDA to finally enforce clinical trial reporting law (13-03-2023). TranspariMED.
Op de hoogte blijven van actueel nieuws, bijeenkomsten en publieke consultaties