Uitgelicht en gespot op internet (week 2, 2022)
Hierbij ontvangt u een overzicht van actualiteiten en berichten die de afgelopen tijd zijn gespot op het internet.
Huishoudelijke mededelingen: de ‘Events‘ pagina is bijgewerkt en voorzien van nieuwe bijeenkomsten. Ook zijn er een aantal hyperlinks in oude berichten gerepareerd die door linkrot niet meer te bereiken waren.
‘Uitgelicht en gespot op internet‘ is een supplement van de Nieuwsbrief voor Goede Onderzoekspraktijken.
Algemene verordening gegevensbescherming (AVG), European Data Protection Board (EDPB)
Biomarkers
- De bodemloze biomarkerput (13-01-2022). NTvG.
- Van beloftevolle biomarker naar nuttige test (13-01-2022). NTvG.
Clinical research, Clinical trials
- Responding to signals of mental and behavioral health risk in pragmatic clinical trials: Ethical obligations in a healthcare ecosystem (februari 2022). Contemporary Clinical Trials.
- Decentralized Clinical Trials Raise Concerns About Informed Consent (11-01-2022). PRWeb.
- The role of community advisory boards in community-based HIV clinical trials: a qualitative study from Tanzania (08-01-2022). BMC Medical Ethics.
- How to Use and Interpret the Results of a Platform TrialUsers’ Guide to the Medical Literature (04-01-2022). JAMA.
- Sharing Clinical Trial Results with Pediatric Participants (16-12-2021). Applied Clinical Trials.
- Racial and Ethnic Representation in US Clinical Trials of New Drugs and Biologics, 2015-2019. JAMA. 2021 Dec 7;326(21):2201-2203.
Clinical Trials Regulation (CTR), Clinical Trials Information System (CTIS)
- Organisation data quality standards in OMS. Data quality principles (06-01-2022). EMA. > Ga naar ‘Documents’ om het document ‘C – OMS Data Quality standards’ te kunnen downloaden.
- Commission Implementing Regulation (EU) 2022/20 of 7 January 2022 laying down rules for the application of Regulation (EU) No 536/2014 of the European Parliament and of the Council as regards setting up the rules and procedures for the cooperation of the Member States in safety assessment of clinical trials (Text with EEA relevance).
- European Commission creates rules for allocating clinical trial safety oversight (12-01-2022). Regulatory Focus.
Dutch Oncology Research Platform (DORP)
European CRO Federation (EUCROF)
European Medicines Agency (EMA)
- Accelerating Clinical Trials in the EU (ACT EU): for better clinical trials that address patients’ needs (13-01-2022).
- ACT EU strategy paper: Accelerating clinical trials in the EU (ACT EU) – Delivering an EU clinical trials transformation initiative (13-01-2022).
Health-RI
Innovative Health Initiative (IHI)
International Society for Biological and Environmental Repositories (ISBER)
Medicines and Healthcare products Regulatory Agency (MHRA)
Misconduct, Fraud, Research integrity
- “Bosom peril” is not “breast cancer”: How weird computer-generated phrases help researchers find scientific publishing fraud (13-01-2022). The Bulletin.
- Scientific journals must be alert to potential manipulation in citations and referencing (13-01-2022). Research Ethics.
- Stamp out fake clinical data by working together (11-01-2022). Nature.
- Misconduct in research administration: What is it? How widespread is it? And what should we do about it? (06-01-2022). Accountability in Research.
- Dialogical teaching of research integrity: an overview of selected methods (23-12-2021). Facets.
Nieuwsbrief voor Goede Onderzoekspraktijken
PGOsupport
NIH Pragmatic Trials Collaboratory
Research reporting
US Food and Drug Administration (FDA)
Wet- en regelgeving
Bezig met het samenstellen van een overzicht van wet- en regelgeving rond (medisch-)wetenschappelijk onderzoek. Hierbij nodig ik anderen uit om input te leveren. Naast de in het document genoemde wet en regelgeving heb ik al wat “regeltjes” in klad staan. Ik ben echter ook zeer benieuwd naar de input van mijn netwerk. Ik hoor het graag van jullie!
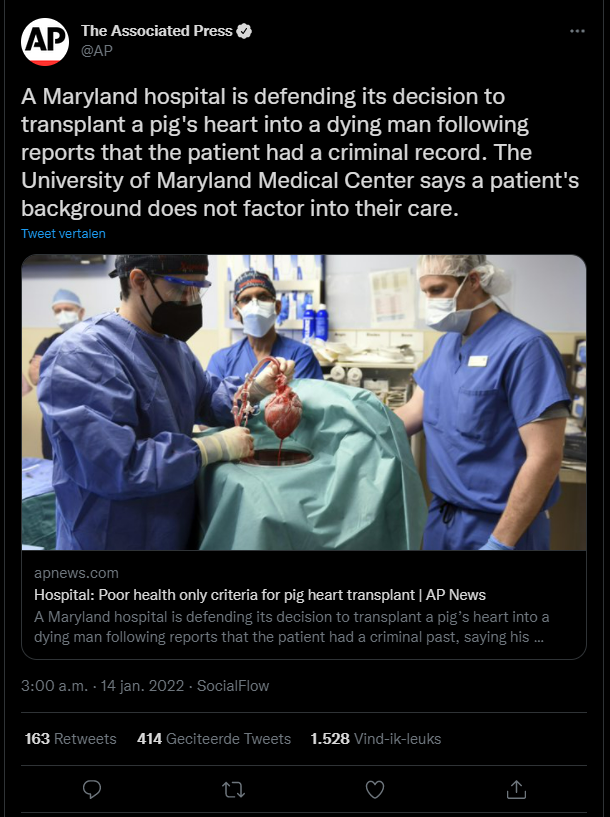
Xenotransplantatie
- Opinion: Here are some questions about the pig heart transplant that people actually should be asking (14-01-2022). Washington Post.
- First transplant of a genetically altered pig heart into a person sparks ethics questions (10-01-2022). STAT.
- In a First, Man Receives a Heart From a Genetically Altered Pig (10-01-2022). The New York Times.
Overige berichten
- Weekend reads: A White House official’s retraction; ‘bosom peril;’ nonsense with a forged authorship (15-01-2022). Retraction Watch.
- Scientists are making CAR-T cells more clever. Here’s what the next generation could look like (14-01-2022). STAT.
- WHO charts path to convergence on cell and gene therapies (14-01-2022). Regulatory Focus.
- Dismantling Systemic Racism and Advancing Health Equity throughout Research (10-01-2022). National Academy of Sciences.
- Unprecedented Decline in One-and-Done PI Rate Leads to Reversal of Turnover Trend (10-01-2022). WCG CenterWatch.
- Challenges in confirming drug effectiveness after early approval. Science. 2021 Dec 3;374(6572):1205-1207.
- The Impact of Variability in Patient Exposure During Premarket Clinical Development on Postmarket Safety Outcomes. Clin Pharmacol Ther. 2021 Dec;110(6):1512-1525.
Op de hoogte blijven van actueel nieuws, bijeenkomsten en publieke consultaties