Uitgelicht en gespot op internet (week 15, 2021)
Hierbij weer een nieuw overzicht van nieuws en andere ontwikkelingen die de afgelopen weken zijn gespot op het internet.
BBMRI-ERIC
Bescherming persoonsgegevens
- Territorial Scope and Data Transfer Rules in the GDPR: Realising the EU’s Ambition of Borderless Data Protection (16 april 2021). University of Cambridge Faculty of Law Research Paper No. 20/2021.
- EDPB cautiously welcomes UK adequacy finding (16 april 2021). LexBlog.
- ‘Breng privacyregels in diverse zorgwetten op één lijn’ (15 april 2021). Medisch Contact.
- International Sharing of Personal Health Data for Research (15 april 2021). COREON.
Centrale Commissie Dierproeven (CDD)
Clinical research,
- FDA: Master protocols have value in COVID-19, and beyond (15 april 2021). Regulatory Focus.
- Study Finds Pandemic Mitigation Efforts Impacted Trial Completion Rates (12 april 2021). WCG CenterWatch.
- Thousands of lives put at risk by clinical trials system that is ‘not fit for purpose’ (31 maart 2021). The Guardian.
- Combining drugs and extending treatment — a PFS end point is not sufficient (23 mei 2017). Nature Reviews Clinical Review.
COVID-19
- EU Covid19 open calls for proposals on Research Infrastructures, vaccines & treatments Clinical Trials and cohorts are now open.
- Cohorts united against COVID-19 variants of concern
- Vaccines & therapeutic clinical trials to boost COVID-19 prevention and treatment
- Research infrastructure services for rapid research responses to COVID-19 and other infectious disease epidemics
- FAIR and open data sharing in support to European preparedness for COVID-19 and other infectious diseases
Drug development, Farmacovigilantie,
- ‘We did need to throw a lot of spaghetti against the Covid window’: a biotech analyst on pandemic drug development (16 april 2021). STAT.
- Next-generation Covid-19 vaccines are supposed to be better. Some experts worry they could be worse (16 april 2021). STAT.
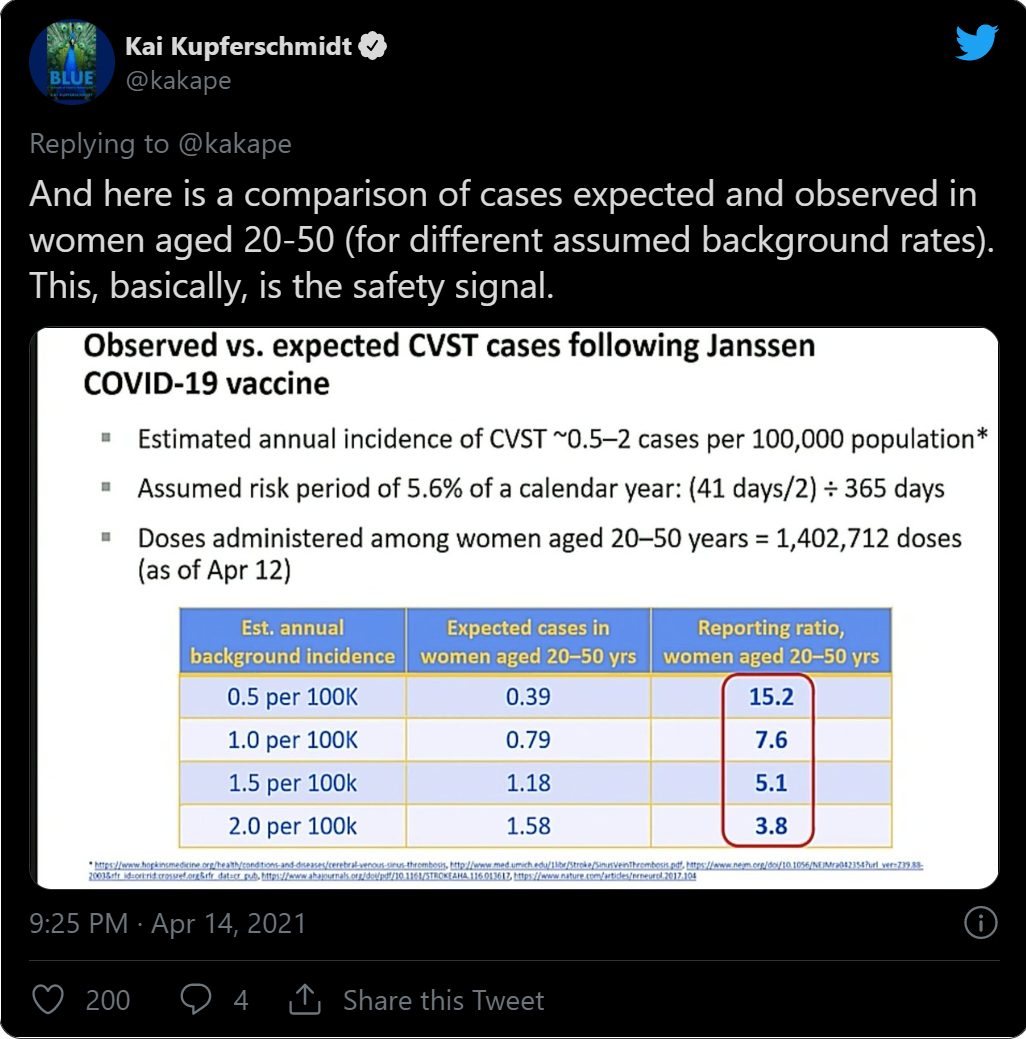
- Cerebral venous thrombosis: a retrospective cohort study of 513,284 confirmed COVID- 19 cases and a comparison with 489,871 people receiving a COVID-19 mRNA vaccine (preprint). OSF, 15 Apr. 2021.
- The J&J Vaccine Pause Is Science Working Like It’s Supposed To (15 april 2021). SLATE.
- I’m a Virus Expert Who Got the J&J Vaccine. I’m Not Losing Sleep. (15 april 2021). The New York Times.
- COVID-19 Vaccine Janssen: assessment of very rare cases of unusual blood clots with low platelets continues (14 april 2021). EMA.
- AstraZeneca’s COVID-19 vaccine: EMA to provide further context on risk of very rare blood clots with low blood platelets (14 april 2021). EMA.
- Joint CDC and FDA Statement on Johnson & Johnson COVID-19 Vaccine (13 april 2021). FDA.
- FDA, CDC recommend J&J vaccine pause while rare clots investigated (13 april 2021). Regulatory Focus.
- U.S. urges pause on use of Johnson & Johnson Covid-19 vaccine after rare blood clotting cases (13 april 2021). STAT.
- U.S. pauses J&J vaccine rollout after 6 people of 6.8 million get rare blood clots (13 april 2021). Science.
- Why would a Covid vaccine cause rare blood clots? Researchers have found clues (13 april 2021). STAT.
- Voorzitter Gezondheidsraad: ‘Het beste voor vertrouwen in vaccins, is volkomen transparant zijn’ (12 april 2021). De Volkskrant.
- Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination (16 april 2021). NEJM.
- Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination (9 april 2021). NEJM.
- Brief report ‘Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination’ (9 april 2021). NEJM.
- Comparing the Covid-19 vaccines developed by Pfizer, Moderna, and Johnson & Johnson (2 februari 2021). STAT.
Embryo onderzoek
- International team creates first chimeric human-monkey embryos (15 april 2021). STAT.
- First monkey–human embryos reignite debate over hybrid animals (15 april 2021). Nature.
Ethics
European Union Drug Regulating Authorities Clinical Trials Database (EudraCT)
European Clinical Research Infrastructure Network (ECRIN)
- Marketplace for scientific clinical research on Covid-19 (12 april 2021).
- ECRIN Newsletter – April 2021 (14 april 2021).
Examenbureau Medisch-Wetenschappelijk Onderzoeker (EMWO)
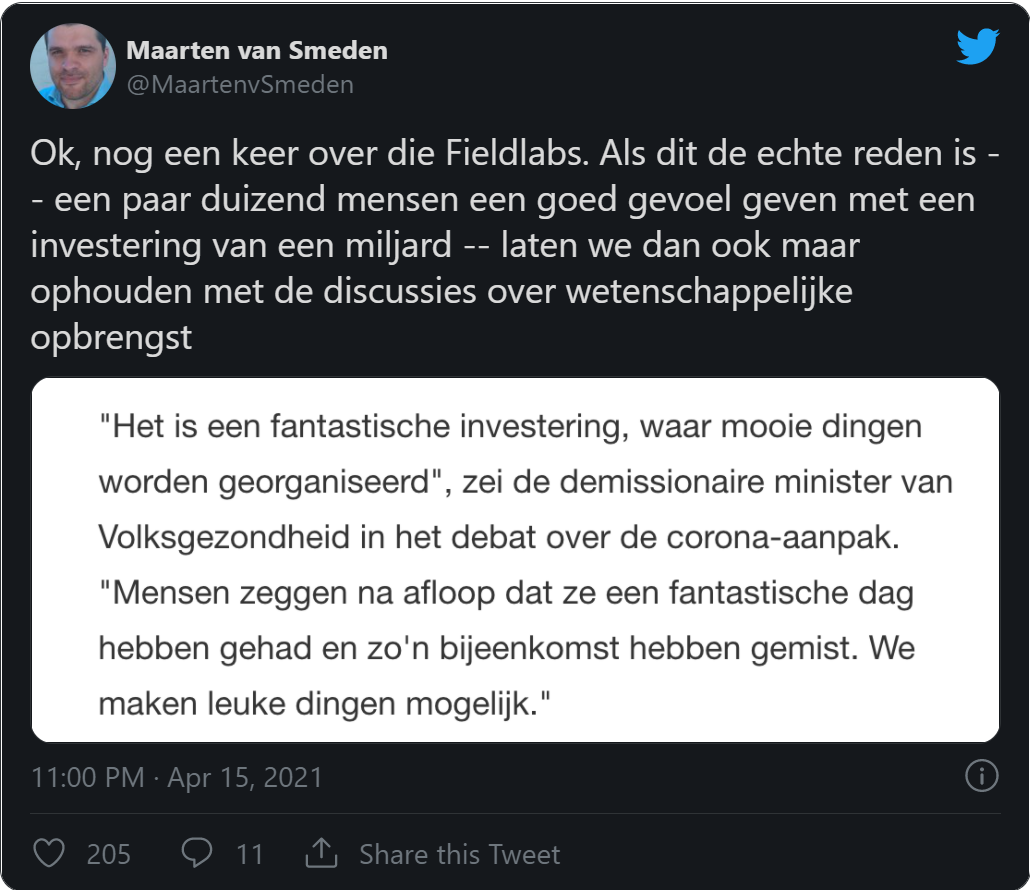
Fieldlab studies
- Kabinet geeft Fieldlab Evenementen toestemming voor opschalen (13 april 2021). Ministerie EZK en OCW.
- Kabinet start pilots toegangstesten (6 april 2021). Ministerie VWS.
- Onderzoeken over gedrag en het coronavirus. Rijksoverheid.
Geneesmiddelenonderzoek, ECTR, CTIS
- Clinical Trials Information System (CTIS): training programme
- Industry Webinar – Introduction to RMS services and activities (15 maart 2021). EMA via YouTube.
- Industry Webinar Introduction to OMS services and activities (15 maart 2021). EMA via YouTube.
Gentherapie
Medische hulpmiddelen, MDR, IVDR
- MDR/IVDR: Commission adopts new standardization request (16 april 2021). Regulatory Focus.
- Verslag NVFG bijeenkomst “Medical Device trials: waar moet ik beginnen?” dinsdag 13 april 2021, door Joost Melis. NVFG.
- Download presentaties NVFG Medical Device Meeting (13 april 2021):
- Gert Bos – MDR en IVDR – “De essentials”
- Annet Muetstege – ISO 14155: 2020, de nieuwe GCP voor medical devices: Waar moet ik op letten?”
- Niels van Tienen – Medical data collection Platforms
- Erik Vollebregt – Artikel 117 MDR en Klinisch onderzoek met combinatie-producten en veranderingen in de juridische parameters
- Download presentaties NVFG Medical Device Meeting (13 april 2021):
- MDR date of application next month – and the book (11 april 2021). Medical devices legal.
Patient involvement
- Device Consortium Pushes for Stronger Patient Voice in Trials (12 april 2021).
- Maximizing Patient Input in the Design and Development of Medical Device Clinical Trials (5 april 2021). Medical Device Innovation Consortium (MDIC).
Platform for European Preparedness Against (Re-)emerging Epidemics
Publications, Preprints
Safety reporting
ZonMw
Overige
- Journal of Clinical Research Best Practices, April 2021.
- Countries ‘must do their part’ in widening EU’s R&D strength (15 april 2021). Research Professional News.
- Why researchers created a database of half a million journal editors (13 april 2021). Nature Index.
- Views on ethical issues in research labs: A university-wide survey (13 april 2021). Accountability in Research.
- A self-correcting fallacy – Why don’t researchers correct their own errors in the scientific record? (13 april 2021). LSE Blog.
- Regulators: Focus Global Harmonization Efforts on Rare Disease Trials (12 april 2021). WCG CenterWatch.
- How to Handle Co-authorship When Not Everyone’s Research Contributions Make It into the Paper (12 april 2021). Science and Engineering Ethics.
- The informed consent process in health research with under-served populations: a realist review protocol (9 april 2021). Systemic Reviews.
- AI uses patient data to optimize selection of eligibility criteria for clinical trials (7 april 2021). Nature.
Raadpleeg het weblog voor een overzicht van het laatste nieuws, bijeenkomsten en publieke consultaties.
Nieuwsfeed
> news.bontrop.com <