Uitgelicht en gespot op internet (week 49, 2023)
Hierbij een nieuwe uitgave van ‘Uitgelicht en gespot op internet‘, een supplement van de ‘Nieuwsbrief voor Goede Onderzoekspraktijken‘, met een overzicht van nieuws en andere berichten die zijn gespot op het internet.
Vincent Bontrop
www.bontrop.com
U kunt met mij linken via LinkedIn.
Accelerating Clinical Trials in the EU (ACT EU)
Belgian Association of Research Ethics Committees (BAREC)
Clinical trials
- New guide, new endpoint: Studying hypoglycaemia for diabetic drug efficacy (08-12-2023). Cinical Trials Arena.
- Navigating Cell Therapy Clinical Trial Budgets: Proactive Strategies for Success (08-12-2023). Clincal Research News.
- 3 Reasons Why Sponsors Must Review Monitoring Reports (06-12-2023). Clinical Leader.
- How Planning For Failure Can Bring Patient Recruitment Success (05-12-2023). Clinical Leader.
- ACRP Addresses Leading Barrier to DCT Adoption at Clinical Trial Sites (04-12-2023). ACRP.
- Opportunities and challenges for decentralized clinical trial approaches: European health technology assessment perspective (01-12-2023). Value in Health.
COMBINE project
Diversiteit, Inclusiviteit, Representativiteit, Recruitment, Retention
European Animal Research Association (EARA)
- Spanish biomedical institutions improve their openness on animal research – new report (05-12-2023).
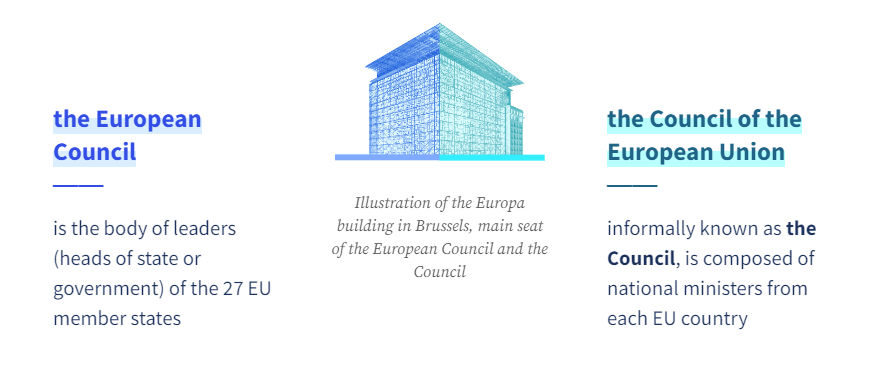
European Council or Council of the EU?
European Health Data Space (EHDS)
- VWS start programma Health Data Access Body (HDAB)-NL (04-12-2023). ministerie van Volksgezondheid, Welzijn en Sport.
- EFPIA response to EHDS report adopted by ENVI/LIBE committee in the European Parliament (01-12-2023). EFPIA.
European Medicines Agency (EMA)
- Frequently asked questions on medicinal products development and assessment involving companion diagnostic (CDx) (06-12-2023).
- Guideline on specific adverse reaction follow-up questionnaires (Specific AR FUQ) (06-12-2023).
- CTIS newsflash – 24 November 2023 (05-12-2023).
Intergraal Kankercentrum Nederland (IKNL)
- Oncode Accelerator van start voor snellere en betere ontwikkeling kankermedicijnen (29-11-2023).
- Overzicht kankerstudies nu ook te zien op de website van het Oncologisch Netwerk Friesland (28-11-2023).
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH)
Kunstmatige Intelligentie (KI)
Nationale Dialoog Klinisch Onderzoek, Nationaal Actie Plan Klinisch Onderzoek, de Benchmark-studie
- Rapport: EU Benchmark Clinical Research. The Netherlands as a clinical trial location in Europe (Oktober 2023) (120 pagina’s).
- Samenvatting: ‘The Netherlands Positioning Within the European Clinical Trials Ecosystem’ (updated 01-10-2023). (67 pagina’s).
- Klinisch onderzoek in Nederland ‘Nederlandse expertise en pioniersgeest internationaal geroemd’ (05-12-2023). DCRF.
- Nederland blinkt – nu nog – internationaal uit in klinisch onderzoek (04-12-2023). DCRF.
Trials@Home
U.S. Food and Drug Administration (FDA)
- Study Data Standards Resources (08-12-2023).
- The Importance of Clinical Trial Transparency and FDA Oversight (04-12-2023).
- FDA warning letters address CGMP, clinical study plan failures (04-12-2023). Regulatory Focus.
ZonMw
Overig
- Weekend reads: NEJM’s racist past; ‘journal editors are like gods’; Harvard accused of pushing out misinformation researcher for criticizing Meta (09-12-2023). Retraction Watch.
- Weekend reads: Hijacked journals polluting an index; special issues take a hit; a data breach at a megajournal (02-12-2023). Retraction Watch.
- Euro Roundup: EMA identifies need to ‘streamline’ processes in wake of COVID-19 pandemic (07-12-2023). Regulatory Focus.
- How to cover academic research fraud and scientic errors (05-12-2023). Journalists Resource.
- How to Stop Academic Fraudsters (04-12-2023). The Chronicle of Higher Education.
- Factors influencing the time to ethics and governance approvals for clinical trials: a retrospective cross-sectional survey (01-12-2023). Trials.
- The quizzical failure of a nudge on academic integrity education: a randomized controlled trial (30-11-2023). Research Integrity and Peer Review.
- Former rector Sijtsma: Turn to statistician to fight fraud and sloppiness (28-11-2023). Tilburg Unversity Magazine.
- 386. Participant Reflections on Their Experiences Participating in Phase 1 and 2 Inpatient Vaccine Trials and Human Challenge Studies (27-11-2023). Open Forum Infectious Diseases.
- Adverse Event Reporting in Randomized Clinical Trials for Multiple Myeloma | Hematology (10-11-2023). JAMA Network Open.