Uitgelicht en gespot op internet (week 11, 2022)
Hierbij een nieuwe uitgave van het supplement ‘Uitgelicht en gespot op internet‘, met een overzicht van nieuws en andere berichten die de afgelopen weken zijn gespot op het internet. Er zijn ook weer nieuwe bijeenkomsten en publieke consultaties toegevoegd aan de ‘Events‘ en de ‘Consultations‘-pagina.
‘Uitgelicht en gespot op internet‘ is een supplement van de ‘Nieuwsbrief voor Goede Onderzoekspraktijken‘.
Centrale Commissie Dierproeven (CCD)
Centrale Commissie Mensgebonden Onderzoek (CCMO), Clinical Trials Regulation (CTR)
De CCMO heeft haar CTR Q&A bijgewerkt en een aantal nieuwe documenten online geplaatst.
- Instructies voor wijzigen applicatie en documentnamen
- Uitleg codes en document titels CTIS indiening
- Vragen en antwoorden CTR – Nederland d.d. 21 februari 2022.
Clinical Trials Information System (CTIS)
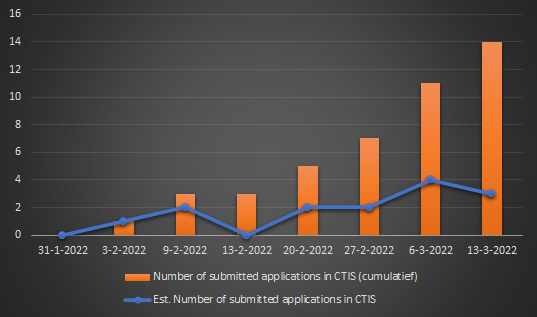
As of 17 March 2022, over 5,000 logins to CTIS had been recorded and sponsors had submitted 14 clinical trial applications for assessment by the relevant regulatory authorities.
bron: EMA Management Board: highlights of March 2022 meeting (18-03-2022).
Niet bekend is of het aantal van 14 ingediende aanvragen in- of exclusief de twee testdossiers is die momenteel worden weergegeven in het CTIS register, klik hier om naar het CTIS zoekresultatenvenster te gaan.
Clinical Trials Transformation Initiative (CTTI)
- CTTI to Launch New Online Digital Health Trials Hub (15-03-2022).
- New CTTI Project Aims to Promote the Use of Disease Progression Modeling to Advance Trial Design and Decision Making (10-03-2022).
Dutch Association of Research Quality Assurance (DARQA)
European Clinical Research Infrastructure Network (ECRIN)
European Medicines Agency (EMA)
- Q&A: Good clinical practice (GCP) (updated March 2022).
- EMA Management Board: highlights of March 2022 meeting (18-03-2022).
- EU GMP Annex 1 revision to be released mid-year (15-03-2022). Regulatory Focus.
- EMA releases draft guidelines on GVP for pregnancy prevention programs (15-03-2022). Regulatory Focus.
- GSK falls in line with Big Pharma’s reaction to Ukraine invasion, cutting off new clinical trials, donating profits and more (17-03-2022). EndpointsNews.
- GlaxoSmithKline says it will not start any new clinical trials in Russia (17-03-2022). The Guardian.
- Eli Lilly Scales Back Clinical Trials, Operations in Russia (15-03-2022). The Wall Street Journal.
…, we will no longer initiate new clinical trials in Russia, and we will stop recruiting new patients in our ongoing clinical trials in the country. Pfizer will work with FDA and other regulators to transition all ongoing clinical trials to alternative sites outside Russia. Consistent with our commitment to putting patients first, we will continue providing needed medicines to the patients already enrolled in clinical trials.
bron: Pfizer Updates Company Position in Russia d.d. 14-03-2022
- Pfizer Updates Company Position in Russia (14-03-2022).
- Pfizer Halts Clinical Trials in Russia But Will Continue to Supply Medicine (14-03-2022). TIME.
- Drugmakers pause trials in Russia while still sending medicine (14-03-2022). Bloomberg.
- The War Puts Ukraine’s Clinical Trials—and Patients—in Jeopardy (14-03-2022). WIRED.
- ICMRA Executive Committee response to situation in Ukraine (13-03-2022). International Coalition of Medicines Regulatory Authorities.
Following a request from an ICMRA member, the Executive Committee met on 2 March 2022 and agreed on the following response. As an immediate step Russian Federal Service for Surveillance in Healthcare (Roszdravnadzor) representatives will no longer be invited to upcoming ICMRA meetings, teleconferences and workstreams, and will no longer be included in information exchanges.
bron: ICMRA Executive Committee response to situation in Ukraine d.d. 13-03-2022.
Oncode Institute
PGOsupport
- Opgeleide ‘critical friends’ nu vindbaar via ikzoekeenpatient.nl (16-03-2022).
- Farmaceutische bedrijven zien EUPATI inmiddels als keurmerk (01-03-2022).
US Food and Drug Administration (FDA), Verenigde Staten
- Remote Data Acquisition in Clinical Trials (15-03-2022). ECA Academy.
- Avoid Injury Reimbursement Quagmire with Comprehensive Policy and Process (14-03-2022). WCG CenterWatch.
- Analysis: FDA’s Five-Year Diversity Strategy Fell Short for Black Patients (14-03-2022). WCG CenterWatch.
- Brace for Quality System-Related Scrutiny During GCP Inspections (14-03-2022). WCG CenterWatch.
ZonMw
Overige berichten
- Weekend reads: False data in Columbia rankings?; data service accused of intimidating researchers; preprint server removes ‘inflammatory’ papers (19-03-2022). Retraction Watch.
- How much do the public sector and the private sector contribute to biopharmaceutical R&D? (april 2022). Drug Discovery Today.
- Drug Discovery Today, Volume 27, Issue 4, Pages 935-1204 (April 2022).
- Wetenschap is beter af zonder ranglijsten (18-03-2022). NRC.
- Researchfish accused of ‘intimidating’ academics (18-03-2022). Research Professional News.
- If pharma can market to youths by TikTok, it should include them in clinical trials (18-03-2022). STAT.
- Kennisveiligheid rondom China nog maar kort op netvlies bij OCW (16-03-2022). ScienceGuide.
- Ethnic minority experts excluded from peer review in UK, MPs told (16-03-2022). Times Higher Education.
- Reproducible brain-wide association studies require thousands of individuals (16-03-2022). Nature.
- UK regulators cooperate to boost clinical trial reporting (maart 2022). TranspariMED.
- An open-access history: the world according to Smits (14-03-2022). Nature.
- Digital Medicine Society to Tackle Technological Barriers in Trials (14-03-2022). WCG CenterWatch.
- Is anonymity or transparency the best solution to bias in peer review? (14-03-2022). Times Higher Education.
- Who Owns CRISPR? Understanding the Confusion Around This Revolutionary Tech (11-03-2022). The Wire.
- How is inclusiveness in health systems research priority-setting affected when community organizations lead the process? (16-02-2022). Health Policy and Planning.
- Let’s end “real-world evidence” terminology usage: A study should be identified by its design (12-11-2021). Journal of Clinical Epidemiology.
Op de hoogte blijven van actueel nieuws, bijeenkomsten en publieke consultaties