Uitgelicht en gespot op internet (week 48, 2020)
Een verzameling van nieuwsberichten die de afgelopen weken zijn gespot op het internet:
COVID-19
- How to manage when your fieldwork is cancelled (27 november 2020). Nature.
- Global regulators urge continuation of COVID-19 vaccine trials for longer-term safety and efficacy follow-up (27 november 2020). EMA.
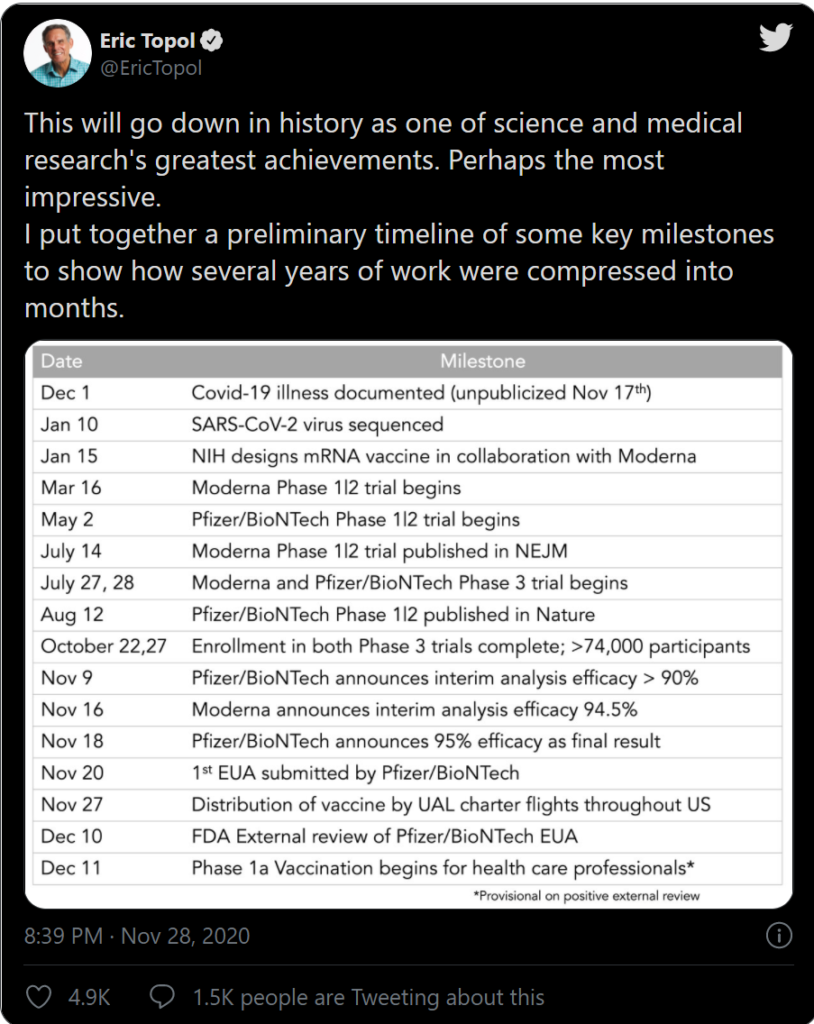
- A timeline of the Oxford-Astrazeneca COVID-19 vaccine trials (25 november 2020). Hilde Bastian.
- The AstraZeneca Covid Vaccine Data Isn’t Up to Snuff (25 november 2020). Wired.
- How to Investigate a Serious Adverse Event Reported During a Clinical Trial for a COVID-19 Vaccine (21 november 2020). Drug Safety.
- Should researchers shelve plans to deliberately infect people with the coronavirus? (20 november 2020). Science.
- Pregnant women haven’t been included in promising COVID-19 vaccine trials (23 november 2020). USA Today.
- Pregnant people haven’t been included in promising COVID vaccine trials (17 november 2020). The 19th.
- The Latest Covid Vaccine Results, Deciphered (10 november 2020). Wired.
- Covid-19 Vaccines With ‘Minor Side Effects’ Could Still Be Pretty Bad (21 juli 2020). Wired.
- The brouhaha around placebo choice in vaccine trials (21 februari 2018). Sciblogs.
EudraCT
“The Frequently Asked Questions document has been completely revised. Sponsors are encouraged to refer to it for questions on EudraCT/EU CTR. ….
The Service Level Agreement for the addition of a new active substance in EudraCT has changed. Now it could take 5-10 days to have a substance added in section D. of the Clinical Trial Application form. … ”
-Bron: EudraCT Public website
European Commission
Good Manufacturing Practices
Heath Research Authority (HRA), Medicines and Healthcare products Regulatory Agency (MHRA) & Information Commissioners Office (ICO)
National Survey on Research Integrity
- Largest ever research integrity survey flounders as universities refuse to cooperate (25 november 2020). Science.
- Meeste universiteiten werken niet mee aan groot integriteitsonderzoek (9 november 2020). DUB.
Persoonsgegevens
- Registratie naar etniciteit maakt onderzoek naar oversterfte mogelijk (26 november 2020). Medisch Contact.
- Wanneer is etnische registratie van patiënten toegestaan onder de AVG? (5 november 2020). SOLV.
- Legitimate interest: a fine balance (26 november 2020). Greet Gysen (EDPB), via LinkedIn.
- What’s New in the EDPB’s Draft Guidelines on Controllers and Processors Under the GDPR? (Part 4) (22 november 2020). LexBlog.
- Wat houdt ‘Het beginsel van de minimale gegevensverwerking’ in? (18 augustus 2020). Politeia.
U.S. Food and Drug Administration ·
Overige berichten
- Pharmafocus (December 2020, Vol. 22 Issue 10).
- Expanded Access Programs, compassionate drug use, and Emergency Use Authorizations during the COVID-19 pandemic (27 november 2020). Drug Discovery Today.
- Psychologist’s paper retracted after Dutch national body affirms misconduct findings (23 november 2020). Retraction Watch.
- Ethics and Privacy in Social Media Research for Mental Health (23 november 2020). Current Psychiatry Reports.
- Advancing development of medicines by academia and non-profit research organizations in the European Union (23 november 2020). Nature Reviews Drug Discovery.
- Poll results on co-authorship of papers using publicly available data (23 november 2020). Dynamic Ecology.
- Bystanders, risks, and consent (25 oktober 2019). Bioethics.
Raadpleeg ook het weblog voor een overzicht van het laatste nieuws, bijeenkomsten en publieke consultaties.
Nieuwsfeed
> news.bontrop.com <